Abstract
Background
Cancer cachexia is characterized by a negative energy balance, muscle and adipose tissue wasting, insulin resistance, and systemic inflammation. Because of its strong negative impact on prognosis and its multifactorial nature that is still not fully understood, cachexia remains an important challenge in the field of cancer treatment. Recent animal studies indicate that the gut microbiota is involved in the pathogenesis and manifestation of cancer cachexia, but human data are lacking. The present study investigates gut microbiota composition, short‐chain fatty acids (SCFA), and inflammatory parameters in human cancer cachexia.
Methods
Faecal samples were prospectively collected in patients (N = 107) with pancreatic cancer, lung cancer, breast cancer, or ovarian cancer. Household partners (N = 76) of the patients were included as healthy controls with similar diet and environmental conditions. Patients were classified as cachectic if they lost >5% body weight in the last 6 months. Gut microbiota composition was analysed by sequencing of the 16S rRNA V4 gene region. Faecal SCFA levels were quantified by gas chromatography. Faecal calprotectin was assessed with enzyme‐linked immunosorbent assay. Serum C‐reactive protein and leucocyte counts were retrieved from medical records.
Results
Cachexia prevalence was highest in pancreatic cancer (66.7%), followed by ovarian cancer (25%), lung cancer (20.8%), and breast cancer (17.3%). Microbial α‐diversity was not significantly different between cachectic cancer patients (N = 33), non‐cachectic cancer patients (N = 74), or healthy controls (N = 76) (species richness P = 0.31; Shannon effective index P = 0.46). Community structure (β‐diversity) tended to differ between these groups (P = 0.053), although overall differences were subtle and no clear clustering of samples was observed. Proteobacteria (P < 0.001), an unknown genus from the Enterobacteriaceae family (P < 0.01), and Veillonella (P < 0.001) were more abundant among cachectic cancer patients. Megamonas (P < 0.05) and Peptococcus (P < 0.001) also showed differential abundance. Faecal levels of all SCFA tended to be lower in cachectic cancer patients, but only acetate concentrations were significantly reduced (P < 0.05). Faecal calprotectin levels were positively correlated with the abundance of Peptococcus, unknown Enterobacteriaceae, and Veillonella. We also identified several correlations and interactions between clinical and microbial parameters.
Conclusions
This clinical study provided the first insights into the alterations of gut microbiota composition and SCFA levels that occur in cachectic cancer patients and how they are related to inflammatory parameters. These results pave the way for further research examining the role of the gut microbiota in cancer cachexia and its potential use as therapeutic target.
Keywords: Cachexia, Weight loss, Inflammation, Pancreatic cancer, Lung cancer, Breast cancer
Introduction
Cancer‐induced cachexia is one of the greatest challenges in the field of cancer treatment. This metabolic syndrome, affecting 50–80% of all cancer patients depending on the tumour type, has severe negative consequences for physical functioning, quality of life, and survival. 1 , 2 Cancer cachexia has a multifactorial background and is characterized by an ongoing loss of skeletal muscle mass that cannot be fully reversed by conventional nutritional support. Its pathophysiology is characterized by a negative protein and energy balance driven by a combination of reduced food intake and abnormal metabolism. 1 , 3
During the last decade, it has been shown that crosstalk between commensal bacteria and the human host is essential for the maintenance of homeostasis. More specifically, the gut microbiota has been demonstrated to modulate energy harvest from the diet, systemic inflammation, gut barrier function, and insulin sensitivity, which are metabolic features found to be altered in cancer cachexia. 4 , 5 In light of these findings, it is not surprising that recent animal data indicate that the gut microbiota might be involved in the pathogenesis of cancer cachexia. 6 , 7 For example, Bindels et al. repeatedly demonstrated that cachexia was associated with profound changes in gut microbiota composition and diversity in mouse models of leukaemia and colon cancer. 5 , 8 , 9 Importantly, different approaches to modulate the intestinal microbiota have been shown to affect experimental cancer cachexia. Lactobacillus supplementation successfully reduced pro‐inflammatory cytokine levels and muscle atrophy in mice. 9 , 10 Similarly, a synbiotic approach consisting of inulin‐type fructans and Lactobacillus reuteri was able to counteract microbial aberrations associated with cancer cachexia and improved gut barrier integrity as well as immune function. 8 In addition, this synbiotic intervention reduced muscle wasting and prolonged survival in tumour‐bearing mice. 8 Other multi‐nutrient interventions including amongst others prebiotic oligosaccharides have also been shown to diminish features of cancer cachexia in mice. 11
Mechanistically, the gut microbiota has been shown to influence muscle metabolism by modulating amino acid availability and through the impact of microbial metabolites on glucose metabolism and muscle glycogen availability. 7 Important microbial metabolites exerting systemic effects are bile acids, branched‐chain fatty acids (BCFAs), and short‐chain fatty acids (SCFA), which are produced by macronutrient degradation and released into the blood. 12 The SCFA acetate, propionate, butyrate, and valerate are generated by microbial fermentation of dietary fibres and are closely linked to metabolic health. 13 Butyrate is particularly important for the maintenance of the intestinal epithelium because it serves as fuel for colonocytes and because it promotes expression of tight junction proteins that fortify the epithelial barrier. 13 Furthermore, SCFA have potent anti‐inflammatory and immune‐modulatory effects by activating G protein‐coupled cell surface receptors and by inhibiting the action of histone deacetylases. 14
Despite the promising preclinical data mentioned earlier, human studies addressing the potential relationship between the gut microbiota and cancer cachexia are currently lacking. We present the first clinical study that explores the gut microbiota, SCFA, and inflammatory parameters in cachectic cancer patients.
Methods
Study population
For this cross‐sectional case–control study, patients with pancreatic cancer, lung cancer, breast cancer, or ovarian cancer were recruited via the outpatient clinics of the Maastricht University Medical Centre (MUMC+) between April 2016 and May 2019. Patients were included at the time of diagnosis, before start of systemic therapy or surgery. By enrolling patients with different tumour types, we were able to study cancers with a generally high prevalence of cachexia (pancreas/lung/ovarian) vs. those with a low prevalence (breast), as well as gastrointestinal (pancreas) vs. non‐gastrointestinal (lung/breast/ovarian) cancers. In order to be eligible, patients had to be older than 18 years and should have a recent cancer diagnosis based on radiology, pathology, or cytology. Exclusion criteria were the use of systemic glucocorticoids <4 weeks before inclusion or antibiotics <3 months before inclusion as well as chemotherapy or radiotherapy before sampling. Patients with an additional distinct cancer type, except for basocellular carcinoma of the skin, were excluded. Partners or relatives >18 years and sharing a household with the patient were included as healthy controls. They were included on the assumption to have similar diet style and lifestyle habits, thereby partly controlling for environmental effects on the gut microbiota. Exclusion criteria were the same as for patients. In total, 107 cancer patients and 76 healthy controls were included. The study was approved by the local medical ethics committee of the MUMC+ under Number 15‐4‐022 and was conducted according to the Declaration of Helsinki and its revisions. All participants and enrolled partners gave written informed consent.
Collection of stool samples and clinical data
Stool samples were collected at one time point before treatment. Subjects received a plastic container (SKL, Lochem, the Netherlands) as well as a stool collection device (Tag Hemi VOF, Zeijen, the Netherlands) to ensure hygienic sampling. Prior to a hospital visit, samples were collected at home by the participants and stored in the refrigerator. Upon arrival in the hospital, samples were aliquoted in 2 mL screw cap tubes and stored at −80°C until further analysis.
In addition, clinical information concerning sex, age, body mass index (BMI), and weight loss in the past 6 months was collected by means of a questionnaire. Patients were subsequently classified as cachectic (>5% weight loss in the last 6 months) or non‐cachectic (≤%5 weight loss in the last 6 months). BMI‐adjusted weight loss was categorized using the grading system described by Martin et al. 15 In short, they identified five distinct grades (0–4) of BMI‐adjusted weight loss, which were associated with significantly different survival (Grade 0: longest survival; Grade 4: shortest survival). Additional laboratory parameters [C‐reactive protein (CRP) and leucocyte counts] were assessed in the context of routine care and were retrieved from the patient's medical records, if available (CRP: N = 35, 19 cachectic and 16 non‐cachectic; leucocyte counts: N = 51, 21 cachectic and 30 non‐cachectic).
Faecal microbiota analysis
Metagenomic DNA from faecal samples was extracted by a combination of repeated bead‐beating and column‐based DNA purification using protocol Q of the International Human Microbiome Standards consortium. 16 In short, 200 mg of frozen faeces was homogenized with 1.0 mL ASL lysis buffer (Qiagen, Venlo, the Netherlands) in 2 mL tubes containing 0.3 g of Ø 0.1 mm sterile zirconia beads (BioSpec, Barlesville, OK). Cell lysis was obtained by incubation at 95°C and repeated mechanical disruption using the Fastprep Homogenizer (MP Biomedicals, Brussels, Belgium). Subsequently, DNA isolation was performed using the QIAamp DNA Stool kit according to the International Human Microbiome Standards protocol. DNA was eluted in a final volume of 200 μl, and DNA concentration was measured using a spectrophotometer (DeNovix, Wilmington, DE). Generation of amplicon libraries and sequencing was performed as previously described. 17 Briefly, the V4 hypervariable region of the 16S rRNA gene was PCR amplified from each DNA sample in duplicate. Pooled amplicons from the duplicate reactions were purified using AMPure XP purification (Agencourt, MA, USA) according to the manufacturer's instructions and quantified by Quant‐iT PicoGreen dsDNA reagent kit (Thermo Fisher Scientific, Landsmeer, the Netherlands). Amplicons were mixed in equimolar concentrations to ensure equal representation of each sample and sequenced on an Illumina MiSeq instrument using the V3 reagent kit.
Analysis of faecal short‐chain fatty acid and branched‐chain fatty acid concentrations
Faecal levels of SCFA (acetate, propionate, butyrate, and valerate) and BCFA (isobutyrate and isovalerate) were assessed in a subgroup of 165 participants of whom sufficient faecal material was available. This subgroup consisted of 94 cancer patients (30 cachectic and 64 non‐cachectic) and 71 healthy controls. Within the group of cancer patients, there were 40 patients with breast cancer, 30 with lung cancer, 21 with pancreatic cancer, and 3 with ovarian cancer.
Faecal levels of SCFA and BCFA were quantified by direct‐injection gas chromatography using a Shimadzu GC2025 gas chromatograph (Shimadzu Corporation, Kyoto, Japan) equipped with a flame ionization detector. 18 , 19 Samples were prepared based on an established protocol 20 (see the Methods section in the Supporting Information for a detailed description). SCFA and BCFA levels were corrected for dry weight. For this purpose, 500 mg of frozen faeces was dried in a vacuum dryer (Eppendorff, Nijmegen, the Netherlands) for five hours.
Assessment of faecal calprotectin
Faecal calprotectin levels were assessed in a subgroup of 168 individuals of whom sufficient faecal material was available, amongst which 30 were cachectic cancer patients, 68 non‐cachectic cancer patients, and 70 healthy controls. We excluded one non‐cachectic lung cancer patient from further analysis, because the calprotectin value (829.0 μg/g) was more than 10‐fold higher compared with the rest of the population, without any clinical explanation.
A total of 100 mg faeces was weighed into a 15 mL tube, and 4.9 mL extraction buffer (0.1 M Tris, 0.15 M NaCl, 1.0 M urea, 10 mM CaCl2·2H2O, 0.1 M citric acid, and 0.5% bovine serum albumin, pH 8.0) was added. 21 After 90 min of mixing, 1 mL of suspension was centrifuged at 10 000 g for 5 min at 4°C, and 700 μl supernatant was transferred into a fresh tube and stored at −80°C. Calprotectin concentrations were measured using a commercially available human faecal calprotectin enzyme‐linked immunosorbent assay (lower detection limit 2.56 μg/g) (Hycult Biotech, Uden, the Netherlands). Faecal calprotectin concentrations are expressed in micrograms of calprotectin per gram of faeces.
Statistical analysis of gut microbiota data
Please consult the Methods section in the Supporting Information for a more detailed description of the data analysis.
Preprocessing
Data demultiplexing, quality and length filtering, merging of paired reads, and clustering into Operational Taxonomic Units (OTUs) at 97% sequence identity were performed using the Integrated Microbial Next Generation Sequencing platform (http://www.imngs.org). 22 All downstream analyses were conducted in the R statistical computing environment (Version 4.0.2). 23
Microbial richness, diversity, and community structure
Selection of an appropriate method and subsequent normalization of OTU count tables were performed as previously described, 24 using variant stabilization by the R package DESeq2. 25 Calculation of α‐diversity (observed species richness and Shannon effective index) and β‐diversity (generalized UniFrac) indices was performed using the Rhea pipeline. 26
Bacterial abundances
To examine potential differences in the relative abundance of bacterial genera between cachectic cancer patients, non‐cachectic cancer patients, and controls, all OTUs were combined that were taxonomically assigned to the same genera or phyla; 111 different genera were detected in the dataset. After filtering for a prevalence threshold of at least 10 counts in at least one sample and a presence threshold (one count) in at least 10 samples, 94 genera were obtained. Count tables were normalized using variant stabilization by DESeq2. 25 We used size factor correction to account for differences in sequencing depth between the samples. DESeq2 was also applied to test for differential abundance of genera and phyla. First, all groups were analysed at once in a likelihood ratio test. Further pairwise comparisons were performed using a Wald test to identify genera or phyla that showed changes in abundance across the specific groups. Results are reported as log2 fold changes and associated adjusted P‐values of the likelihood ratio test (Benjamini–Hochberg correction for the number of taxa and in addition the number of groups for the Wald test). Dendrograms were obtained by hierarchical clustering using Ward's method where 1‐Pearson's correlation was used as the distance measure. Composition plots were obtained by transforming the normalized phyla abundances to relative data and next plotting the mean relative abundances per groups using the R package ggplot2 (geom_bar). Correlation analysis was performed using the R package PerformanceAnalytics. 27 Differential co‐occurrence networks were estimated using the R package MDiNE for 1000 Monte Carlo iterations. 28
Statistical analysis of clinical data, short‐chain fatty acid levels, and inflammatory parameters
Statistical analysis was performed using R Version 4.0.0. 23 Depending on whether variables were normally distributed or not, means (±standard deviation) or medians [±inter‐quartile range (IQR)] are reported, and two‐sided Mann–Whitney U test, one‐way analysis of variance, or the Kruskal–Wallis test was applied to assess differences between groups. If the Kruskal–Wallis test revealed significant differences, Dunn's multiple comparison test was used for post hoc analysis and P‐values were adjusted with the Benjamini–Hochberg method. 29 To investigate correlations between different variables, Kendall's tau was used. For assessing potential differences in the distribution of cachectic vs. non‐cachectic patients over disease stages, a χ 2 test was performed. P‐values <0.05 were considered statistically significant.
Results
Baseline characteristics of the study population and prevalence of cancer cachexia
In total, 107 cancer patients and 76 healthy controls participated in the study. Twenty‐seven patients were diagnosed with pancreatic cancer, 52 patients with breast cancer, 24 patients with lung cancer, and 4 patients with ovarian cancer. The majority of patients with breast cancer (96%) or lung cancer (46%) had local disease, whereas the majority of pancreatic cancer patients had lymph nodes involved (59%) or metastatic disease (14%) (Table S2a).
Thirty‐three patients (30.8%) had >5% weight loss in the past 6 months and were classified as cachectic. The prevalence of cancer cachexia varied per cancer type, with the highest prevalence in pancreatic cancer (66.7%), followed by ovarian cancer (25%), lung cancer (20.8%), and breast cancer (17.3%). In cachectic as well as non‐cachectic cancer patients, most patients had local disease (48% in cachectic and 74% in non‐cachectic patients). Lymph node involvement and metastatic disease were more common in the cachectic group, although the distribution of cachectic and non‐cachectic patients over the different disease stages did not differ significantly (Table S2b).
While age (P = 0.287) and BMI (P = 0.055) were not significantly different, weight loss during the past 6 months differed markedly between groups (P < 0.001) and was highest in cachectic cancer patients (8.0 ± 3.0% vs. 0.0 ± 1.7% for non‐cachectic patients, Table 1).
Table 1.
Clinical characteristics of the study population
| Cachectic (N = 33) | Non‐cachectic (N = 74) | Healthy controls (N = 76) | |
|---|---|---|---|
| Age (years), mean ± SD | 65.3 (±12.1) | 61.9 (±10.4) | 62.9 (±9.4) |
| BMI (kg/m2), median ± IQR | 24.3 (±4.8) | 25.5 (±5.1) | 26.5 (±4.1) |
| Weight loss (%), median ± IQR | 8.0 (±3.0) | 0.0 (±1.7) | 0.0 (±0.0) |
| Female | N = 20 (60.6%) | N = 61 (82.4%) | N = 22 (28.9%) |
| Cancer type | |||
| Pancreatic cancer | N = 18 (54.5%) | N = 9 (12.2%) | |
| Breast cancer | N = 9 (27.3%) | N = 43 (58.1%) | |
| Lung cancer | N = 5 (15.2%) | N = 19 (25.7%) | |
| Ovarian cancer | N = 1 (3.0%) | N = 3 (4.1%) | |
BMI, body mass index; IQR, inter‐quartile range; SD, standard deviation.
Variables with a normal distribution are presented as mean ± SD. Variables that were not normally distributed are presented as median ± IQR.
Similar microbial diversity and community structure in cachectic and non‐cachectic patients
First, we determined α‐diversity, reflecting the within‐sample taxonomic diversity. The observed species richness as well as the Shannon effective index, both measures of microbial α‐diversity, were not significantly different in cachectic cancer patients vs. non‐cachectic cancer patients or healthy controls (Figure 1).
Figure 1.
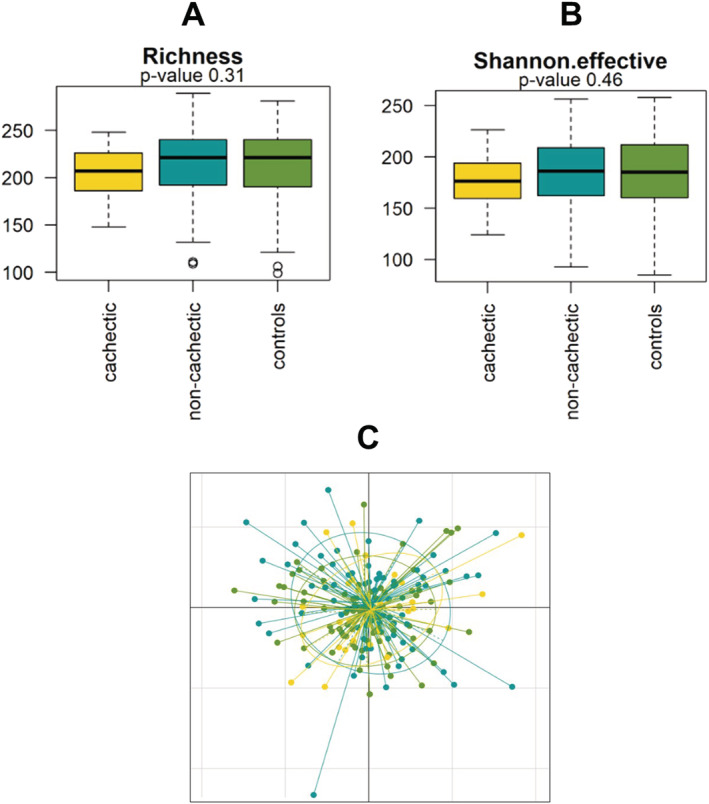
Microbial richness and diversity in cachectic cancer patients (yellow, N = 33), non‐cachectic cancer patients (blue, N = 74), and healthy control subjects (green, N = 76). (A) Observed species richness and (B) Shannon effective index; both indices of α‐diversity were similar between the groups. (C) The non‐metric multidimensional scaling plot showed no clear clustering of samples from cachectic cancer patients, non‐cachectic cancer patients, or healthy controls.
Next, (dis)similarities in microbial community structure (β‐diversity) using generalized UniFrac distances were assessed. Whereas permutational multivariate analysis of variance revealed borderline significant differences in microbial community structure (P = 0.053), the non‐metric multidimensional scaling plot demonstrated that these differences were subtle and no clear clustering of samples was apparent (Figure 1C). Dendrograms also showed no distinct clustering patterns based on cachexia status or BMI and revealed high inter‐individual variability in all study groups (Figure S1).
Distinct gut microbiota composition in cachectic cancer patients
Firmicutes were the most abundant bacterial phylum in all groups, followed by Bacteroidetes, Actinobacteria, and Proteobacteria (Figure 2A). Bacteria belonging to the phylum of Proteobacteria were significantly more abundant in cachectic cancer patients (median log2 abundance = 9.5, IQR = 3.0) when compared with non‐cachectic patients (median log2 abundance = 9.0, IQR = 1.2) and healthy controls (median log2 abundance = 8.8, IQR = 1.2) (P < 0.001) (Figure 2B).
Figure 2.
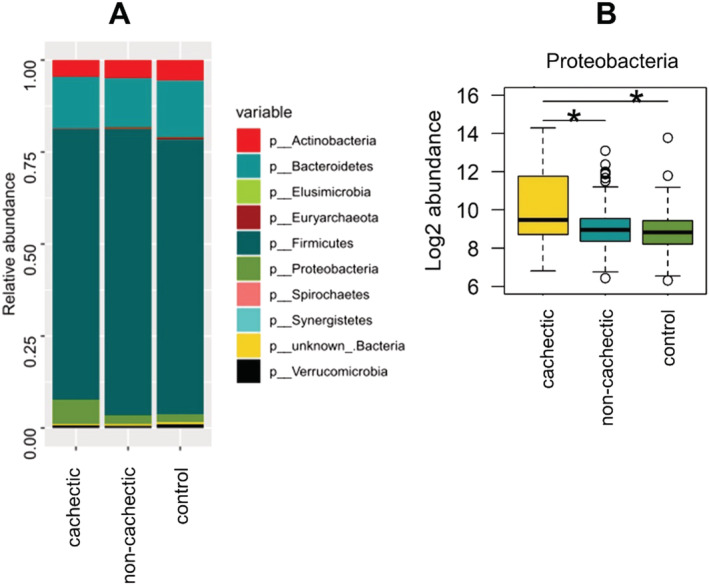
Microbiota composition on phylum level. (A) Relative abundances of all phyla present in the study population. (B) Log2 abundance of Proteobacteria. Statistically significant differences according to the Wald test (α = 0.05) are marked with asterisks. Proteobacteria were significantly elevated in cachectic cancer patients compared with non‐cachectic cancer patients and healthy controls.
On the genus level, the abundances of Megamonas, Peptococcus, Veillonella, and an unknown genus from the Enterobacteriaceae family were found to be significantly different in cachectic cancer patients (Figure 3). For Megamonas and Peptococcus, all medians were zero. While these genera were present in only few samples within the cachectic group, they were more often detected in the non‐cachectic and control groups (Megamonas: P < 0.05; Peptococcus: P < 0.001). With a median log2 abundance of 5.4 (IQR = 10.0), unknown Enterobacteriaceae were much more abundant in cachectic cancer patients compared with non‐cachectic cancer patients (median = 0.69, IQR = 2.1) and healthy controls (median = 0.73, IQR = 1.8) (P < 0.01). Similarly, log2 abundance of Veillonella was highest in cachectic cancer patients (median = 3.2, IQR = 3.5) and significantly lower in non‐cachectic cancer patients (median = 2.3, IQR = 2.7) and healthy controls (median = 1.7, IQR = 3.4) (P < 0.001). No significant differences were found between non‐cachectic cancer patients and healthy controls.
Figure 3.
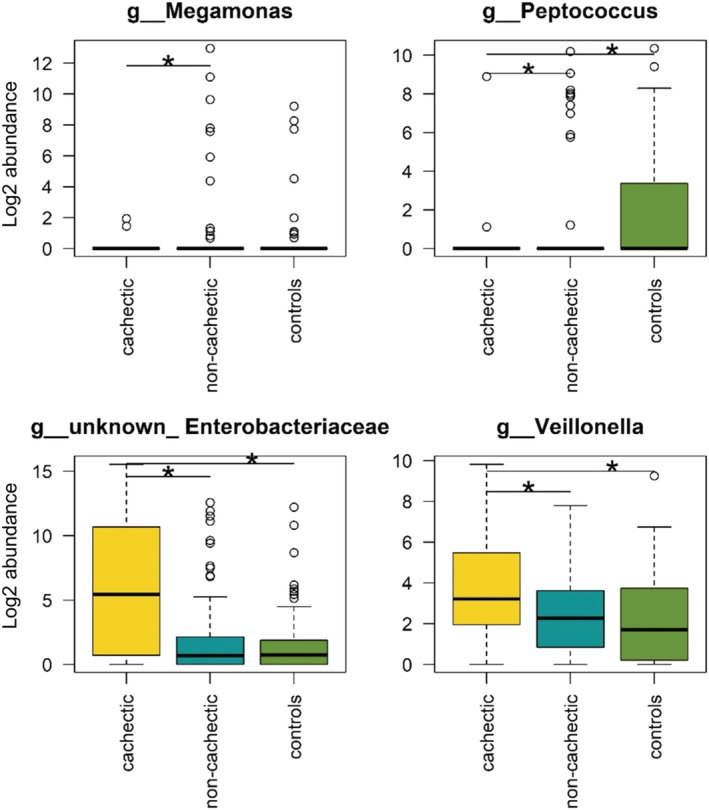
Genera with altered abundance in cachectic vs. non‐cachectic cancer patients and/or healthy controls. The log2 abundance of genera, which differed significantly between the groups, is depicted. Statistically significant differences according to the Wald test (α = 0.05) are marked with asterisks.
Because >5% weight loss might have different clinical relevance in underweight, normal weight, or overweight individuals, we also analysed differential genera abundance in different categories of BMI‐adjusted weight loss, yielding similar results (Figure S2).
Additionally, we analysed differential abundances in pancreatic cancer and lung cancer separately, because cancer cachexia was most prevalent in these two cancer types. These analyses revealed that Enterococcus, Lactobacillus, unknown Enterobacteriaceae, and Veillonella showed differential abundance between cachectic cancer patients, non‐cachectic cancer patients, and healthy controls (Figure S3).
Lower faecal acetate levels in cachectic cancer patients
Total faecal SCFA concentrations tended to be lower in cachectic cancer patients (median = 38.6 mM/g, IQR = 27.0 mM/g) compared with non‐cachectic cancer patients (median = 48.8 mM/g, IQR = 56.1 mM/g) and healthy controls (median = 52.1 mM/g, IQR = 51.4 mM/g) (P = 0.08, Figure 4). The same pattern, with a tendency towards lower levels in the cachectic group, could also be observed when analysing the different SCFA separately (Figure 4). Acetate concentrations were significantly lower in cachectic cancer patients (P < 0.05). Post hoc analysis with Benjamini–Hochberg correction revealed a significant difference between cachectic and non‐cachectic cancer patients (P = 0 < 0.05), but not between cachectic cancer patients and healthy controls (P = 0.059) or non‐cachectic cancer patients and healthy controls (P = 0.62). Faecal concentrations of propionate, butyrate, and valerate were consistently, but not significantly lower in cachectic vs. non‐cachectic cancer patients or healthy controls (Figure 4).
Figure 4.
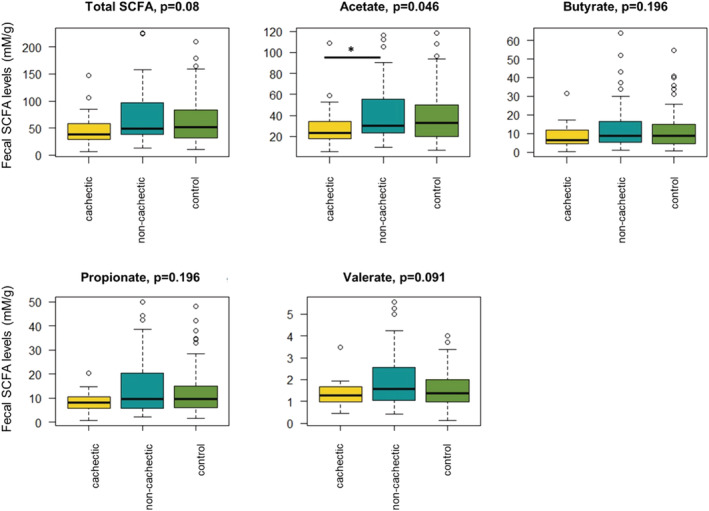
Faecal levels of total short‐chain fatty acids (SCFA) and acetate, butyrate, propionate, and valerate separately. Acetate levels were found to be reduced in cachectic cancer patients (N = 30) compared with non‐cachectic cancer patients (N = 64) and healthy controls (N = 71). P‐values from Kruskal–Wallis test are shown.
We also compared the groups of cachectic cancer patients, non‐cachectic cancer patients, and healthy controls in pancreatic cancer and lung cancer separately, because cancer cachexia was most prevalent in these cancer types. Interestingly, acetate concentrations were only significantly lower within the group of pancreatic cancer patients, while there were no differences in patients with lung cancer. In addition, total faecal SCFA and butyrate concentrations tended to be reduced in cachectic pancreatic cancer patients (Figure S4).
Faecal levels of the BCFA isobutyrate (P = 0.608) and isovalerate (P = 0.543) were similar in cachectic and non‐cachectic cancer patients and healthy controls (Figure S5).
Similar faecal calprotectin levels in cachectic and non‐cachectic cancer patients
Faecal levels of calprotectin, a marker of intestinal inflammation, were not significantly elevated in cachectic cancer patients (median = 51.6 μg/g, IQR = 121.2), compared with non‐cachectic cancer patients (median = 32.1 μg/g, IQR = 37.5) and healthy controls (median = 33.5 μg/g, IQR = 52.1) (P = 0.2, Figure 5).
Figure 5.
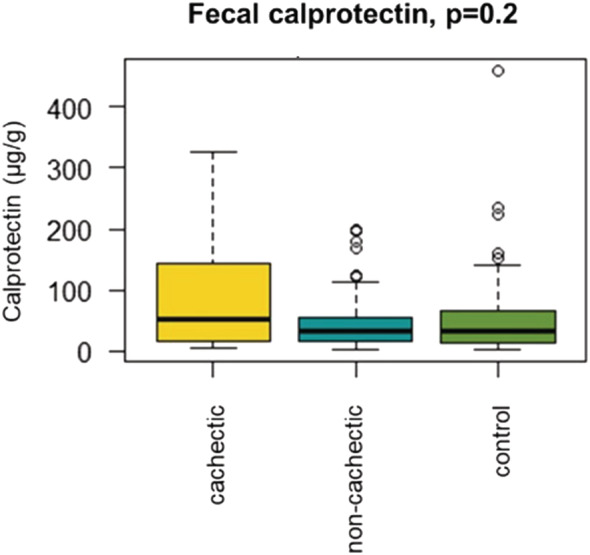
Faecal levels of calprotectin were not different in cachectic cancer patients (N = 30) compared with non‐cachectic cancer patients (N = 68) or healthy controls (N = 70).
CRP levels (P = 0.32, N = 35) and leucocyte counts (P = 0.66, N = 51) were also not significantly different between cachectic and non‐cachectic cancer patients (CRP: median = 7.0 mg/L, IQR = 14.5 in cachectic cancer patients vs. median = 5.0 mg/L, IQR = 9.5 in non‐cachectic cancer patients; leucocyte counts: median = 7.7 × 109/L, IQR = 3.4 in cachectic cancer patients vs. median = 7.9 × 109/L, IQR = 3.4 in non‐cachectic cancer patients).
While there was a strong positive correlation between CRP and leucocyte counts (τ = 0.52, P < 0.001), there were no associations between faecal calprotectin and CRP (τ = 0.14, P = 0.3) or faecal calprotectin and leucocyte counts, respectively (τ = 0.05, P = 0.6). SCFA levels were also not associated with any of these inflammatory parameters (Table S1).
Correlations and co‐occurrences between bacterial taxa, short‐chain fatty acid, calprotectin, and clinical parameters
Next, we performed correlation analysis using the parameters that were found to be significantly different in cachectic vs. non‐cachectic cancer patients according to the previous analyses (Figure 6). We also included faecal calprotectin because we were interested in associations between faecal calprotectin and abundance of specific bacterial taxa. BMI was included because it was almost significant (P = 0.055).
Figure 6.
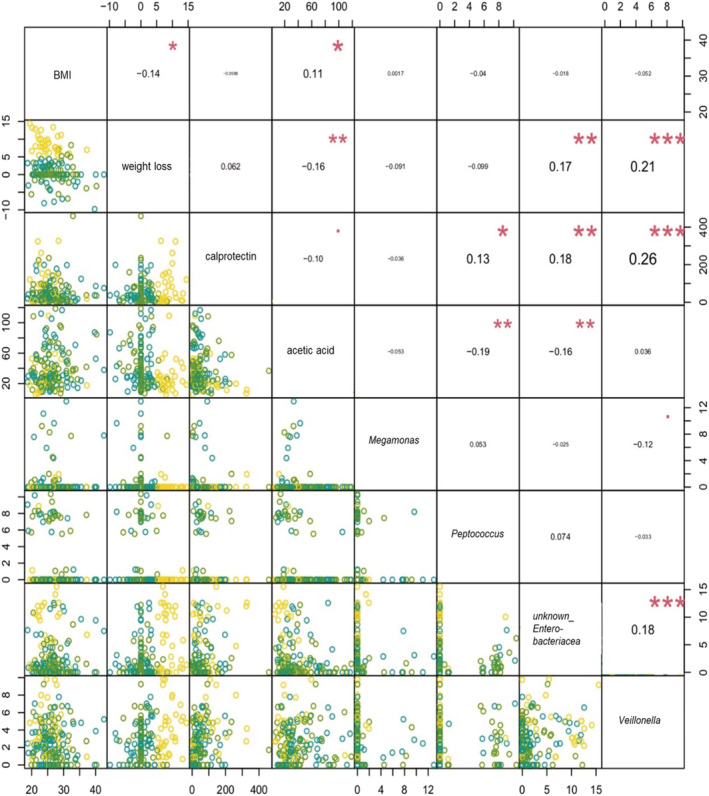
Correlation analyses of the significant variables from differential analyses of bacterial taxa and total short‐chain fatty acids as well as relevant clinical parameters in pairwise comparisons. Factors under investigation are depicted in the diagonal line. The relationships of abundances of four bacterial taxa, acetic acid, calprotectin, body mass index (BMI), and weight loss were estimated using Kendall's tau correlation coefficient (τ). In the upper panels, significant correlations are indicated with asterisks (* P < 0.05, ** P < 0.01, and *** P < 0.001). In the lower panels, scatter plots of pairwise correlations are shown. Yellow dots represent cachectic cancer patients, blue dots depict non‐cachectic cancer patients, and green dots indicate healthy controls.
We found that faecal acetate concentrations were positively correlated with BMI and negatively correlated with weight loss. In addition, faecal acetate was negatively associated with the abundance of Peptococcus and unknown Enterobacteriaceae (Figure 6). Faecal calprotectin levels were positively correlated with the abundance of Peptococcus, unknown Enterobacteriaceae, and Veillonella. Furthermore, we identified strong positive correlations between weight loss and unknown Enterobacteriaceae and Veillonella, respectively. Besides, there was a positive correlation between the abundance of unknown Enterobacteriaceae and Veillonella (Figure 6).
Estimated co‐occurrences confirmed the interaction between unknown Enterobacteriaceae and Veillonella, but showed that the direction of the interaction depended on the group. Within the group of cachectic cancer patients, there was a negative association, potentially indicating competition between these bacteria. While there was a positive interaction in the group of non‐cachectic cancer patients, there was no interaction in healthy controls (Figure 7).
Figure 7.
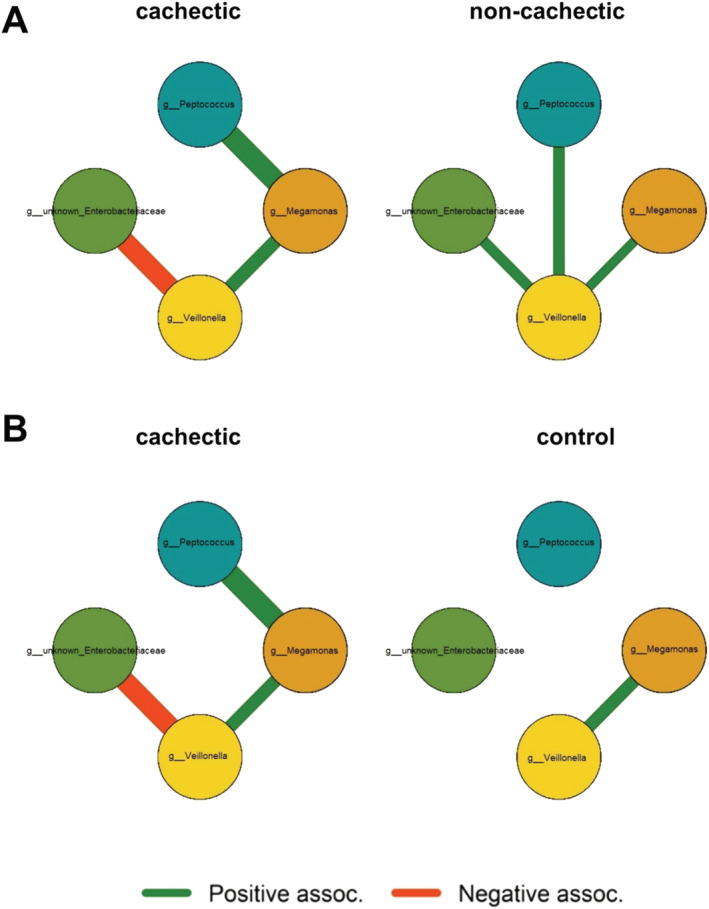
Estimated co‐occurrence of Megamonas, Peptococcus, Veillonella, and an unknown genus from the Enterobacteriaceae family in cachectic cancer patients vs. non‐cachectic cancer patients (A) and cachectic cancer patients vs. healthy controls (B). Green lines represent a positive association, while orange marks a negative association between these genera. An edge is displayed if the 90% credibility interval does not contain zero.
Discussion
This study is the first to provide insights into gut microbiota composition, SCFA levels, and their relationship with inflammatory parameters in the context of human cancer cachexia. We found that overall microbial diversity (α‐diversity) and community structure (β‐diversity) were not altered in patients with cancer cachexia. However, Proteobacteria, an unknown genus from the Enterobacteriaceae family, and Veillonella were found to be more abundant among cachectic cancer patients, and these genera were strongly positively correlated with weight loss. Conversely, the genera Megamonas and Peptococcus showed higher abundance in non‐cachectic patients. In addition, faecal acetate levels were lower in cachectic cancer patients. Whereas faecal calprotectin, a marker of intestinal inflammation, correlated strongly with the abundance of specific gut bacteria, it was not significantly elevated in cachectic cancer patients.
Based on preclinical data showing reduced microbial diversity and altered community structure in cachectic mice, 8 , 30 we expected that α‐diversity and β‐diversity would be reduced in cachectic cancer patients. Furthermore, a species‐rich and diverse microbial community is generally considered to be more healthy and decreased microbial diversity has been linked to several disease states. 31 However, we found no differences in overall microbial diversity and no community pattern that could be linked to the cachectic phenotype in our study population. Nevertheless, differential abundances of certain bacterial taxa were observed in cachectic cancer patients compared with non‐cachectic cancer patients and healthy controls, which corroborate previously reported animal data. Specifically, in line with results from Pötgens et al., who described an expansion of Proteobacteria at the expense of Firmicutes in cachectic C26 mice, 32 the abundance of Proteobacteria was found to be increased in cachectic cancer patients in the current study. In addition, previous studies repeatedly described an increased abundance of Enterobacteriaceae, also belonging to the phylum of Proteobacteria, in cachectic mice. 5 , 8 , 32 We confirm that these cachexia‐associated increased levels of Enterobacteriaceae are also characteristic of human cancer cachexia.
The facultative anaerobic Enterobacteriaceae are considered to be well adapted to survive in environments with high levels of oxidative stress and have been shown to be associated with inflammation. 33 , 34 Interestingly, a shift towards more aerotolerant taxa such as Enterobacteriaceae has also been found in other diseases sharing metabolic and inflammatory features with cancer cachexia, including Crohn's disease 33 and anorexia. 35 Another interesting finding of our study was the co‐abundance of unknown Enterobacteriaceae and Veillonella, which were both increased in cachectic cancer patients. These genera were also significantly correlated with weight loss, confirming their relevance in the context of cancer cachexia. However, it remains to be investigated why the direction of the relationship was different in cachectic vs. non‐cachectic cancer patients. It might be speculated that the increased abundance of unknown Enterobacteriaceae and Veillonella in cachectic cancer patients leads to competition between those bacteria.
Megamonas and Peptococcus, both belonging to the Firmicutes phylum, were only present in a few samples within the cachectic group. This might correspond to the earlier described decreased levels of Firmicutes in cachectic mice, 32 but the total prevalence of these genera in the current study population was too low to draw definite conclusions.
According to our knowledge, this is the first clinical study investigating faecal SCFA concentrations in cachectic cancer patients. SCFA are interesting metabolites in the context of cancer cachexia because they exert anti‐inflammatory effects by interacting with the immune system and by improving gut barrier integrity. 14 , 36 Of note, gut barrier integrity has also been shown to be debilitated in cachectic mice. 5 In addition, it was recently shown that acetate and butyrate were reduced in the caecal content of cachectic mice. 30 In line with this, we observed an overall trend towards lower SCFA concentrations in cachectic cancer patients, although this was only significant for acetate. Acetate is considered to fulfil crucial physiological roles and has been linked to body weight regulation, energy expenditure, lipid metabolism, and insulin sensitivity. 37 Because it has been demonstrated that acetate affects muscle and adipose tissue metabolism, 37 it might be speculated that altered acetate levels could influence cachexia‐associated metabolic disturbances in these target tissues.
We found no associations between faecal SCFA and inflammatory parameters (faecal calprotectin, CRP, and leucocyte counts), which would be expected based on the anti‐inflammatory potential of SCFA. Similarly, we hypothesized that levels of faecal calprotectin would be increased in cachectic cancer patients, because it has been shown to reflect inflammation in the gastrointestinal tract and is useful as biomarker for inflammatory bowel disease and other inflammatory conditions. 38 It was also unexpected that we could not detect differences in CRP levels and leucocyte counts between cachectic and non‐cachectic cancer patients. However, because these inflammatory markers could only be determined in 35 and 51 patients, respectively, and because CRP was elevated (>5 mg/L) in most of them, the relevance of these values in the current study might be limited. Of note, faecal calprotectin strongly correlated with unknown Enterobacteriaceae and Veillonella and to a lesser extent with Peptococcus abundance. The association with unknown Enterobacteriaceae and Veillonella supports the earlier described hypothesis that these bacteria are well adapted to live in a pro‐inflammatory environment.
There are several limitations inherent to this study. First, the prevalence of cancer cachexia varied depending on the cancer type. Consequently, the group of cachectic cancer patients mainly consisted of pancreatic cancer patients, while the majority of the non‐cachectic group had breast cancer. Therefore, differences between cachectic and non‐cachectic cancer patients might be related to the cancer type, next to the presence of cachexia. However, we could partly confirm our results when analysing the different cancer types separately, despite the limitations of small group sizes in these analyses. Nevertheless, future studies should use larger and more homogeneous patient cohorts in order to study crosstalk and mechanistic interactions between gut microbiota and metabolic target tissues in human cancer cachexia in more detail. For instance, it would be beneficial to evaluate differences between cachectic and non‐cachectic cancer patients within one cancer type to rule out the confounding effect of the tumour type.
Besides the cancer type, also the stage of the disease might have influenced microbiota composition. Because lymph node involvement and metastatic disease were more common among cachectic cancer patients, we cannot rule out that the observed microbial disturbances in cachectic cancer patients might also be associated with more advanced stages. In view of the fact that cancer cachexia is considered to be a feature of especially advanced disease stages, it would be highly relevant to compare cachectic and non‐cachectic cancer patients with metastatic disease in future studies. In the metastatic setting, also the role of therapy‐induced cachexia will be important to investigate, because a recent metabolomics study indicated distinct metabolic derangements of cancer‐induced cachexia and chemotherapy‐induced cachexia. 39 Because chemotherapy is known to also affect gut bacteria, the gut microbiota might play a prominent role in this type of cachexia. 40
In addition, the definition of cancer cachexia was solely based on self‐reported weight loss, which might be unreliable and does not take other hallmarks of cancer cachexia (e.g. inflammation and muscle wasting) into account. 41 In addition, it should be recognized that this cachexia definition does not differentiate between loss of skeletal muscle and fat mass and may therefore underestimate lean body mass loss in overweight patients and in those who gained weight because of a tumour, oedema, or accumulation of ascites. 42 Consequently, future studies should use more objective, quantitative methods to assess cachexia status, for instance, computed tomography‐based body composition analysis, and should also assess systemic inflammation in these patients. Another possibility to control for obesity as factor masking relevant weight loss is the classification of patients into different categories of BMI‐adjusted weight loss (Figure S2). 15
In summary, we have shown that cachexia is associated with specific alterations in gut microbiota composition and faecal SCFA concentrations in cancer patients. These insights represent a pivotal first step and underscore the need to evaluate whether the gut microbiota can be used as therapeutic target in cancer cachexia. In view of the limited effectiveness of the current treatment approaches and the considerable impact of cancer cachexia on the patient's prognosis and quality of life, such innovative anti‐cachexia strategies are urgently required.
Author contributions
J.U., D.P.J.v.D., and S.S.R. designed the study. J.U. and D.P.J.v.D. coordinated inclusion, sample collection, and study administration. J.U., J.Z., R.A., D.P.J.v.D., and L.C. included patients. M.L.S., R.F.P.M.K., and S.W.M.O.D. supported inclusion. Laboratory analyses were conducted by J.U., J.Z., and R.A. J.P. and A.v.H. provided resources for laboratory analyses. Bioinformatic and statistical analysis was performed by Z.S. and J.Z. J.U., J.Z., Z.S., R.A., J.P., A.v.H., and S.S.R. were involved in data interpretation. J.Z. and S.S.R. drafted the manuscript. All authors participated in discussion and revision and approved the final version of the manuscript.
Conflict of interest
R.A. and M.L.S. have received institutional research funding from Servier, all outside the submitted work. D.P.J.v.D. was supported by the Netherlands Organization for Scientific Research (NWO Grant 022.003.011). A.v.H. is employed by Danone Nutricia Research. All other authors have no conflicts of interest.
Funding
Danone Nutricia Research contributed financially to the analysis of stool samples.
Supporting information
Table S1. Correlations between SCFA and inflammatory parameters.
Table S2a. Disease stages per cancer type. Information on tumor stages was retrieved from medical records (if available*) and classified as local disease, lymph node involvement, or metastatic disease.
Table S2b. Disease stages in cachectic and non‐cachectic cancer patients. Information on tumor stages was retrieved from medical records (if available*) and classified as local disease, lymph node involvement or metastatic disease.
Figure S1. Dendrograms show no clustering of samples based on cachexia status (upper panel) or BMI (lower panel). We observed high inter‐individual heterogeneity.
Figure S2. Genera abundance across different grades of BMI‐adjusted weight loss. This categorization into grade 0 – grade 4 was suggested in Martin et al. (2015). Diagnostic criteria for the classification of cancer‐associated weight loss. Journal Clinical Oncology, 2015. 33(1): p. 90–9. Each grade was associated with significantly different survival, whereby a gradient of decreasing survival was observed with increasing weight loss and decreasing BMI (grade 0 = longest survival; grade 4 = shortest survival).
Figure S3. Log2 abundance of genera which were differentially abundant between cachectic cancer patients, non‐cachectic cancer patients, and healthy controls, analyzed for pancreatic cancer and lung cancer patients separately. A: The likelihood ratio test (LRT) indicated significant differential abundance of Enterococcus (p < 0.001), Lactobacillus (p < 0.01), unknown Enterobacteriaceae (p < 0.001) and Veillonella (p < 0.001) between cachectic and non‐cachectic pancreatic cancer patients and healthy controls. Differences which were significantly different according to the Wald test (α = 0.05) are marked with asterisks. B: LRT indicated that only Lactobacillus (p < 0.001) showed differential abundance in cachectic and non‐cachectic lung cancer patients and healthy controls. Differences which were significantly different according to the Wald test (α = 0.05) are marked with asterisks.
Figure S4. SCFA levels in patients with pancreatic cancer or lung cancer. A: Acetate levels were significantly lower in cachectic pancreatic cancer patients (N = 16) compared to non‐cachectic pancreatic cancer patients (N = 5). Total SCFA and butyrate concentrations tended to be reduced in cachectic patients. B: Levels of all SCFA were similar in cachectic (N = 5) and non‐cachectic lung cancer patients (N = 25).
Figure S5. Fecal levels of the BCFA iso‐butyrate and iso‐valerate in cachectic cancer patients (N = 30), non‐cachectic cancer patients (N = 64), as well as in healthy controls (N = 71). Levels of iso‐butyrate (p = 0.608) and iso‐valerate (p = 0.543) were similar in all groups.
Acknowledgements
We gratefully thank all volunteers who participated in this study. Furthermore, we are thankful to C. Driessen, M. Oomen, and A. van Bijnen for performing laboratory analyses and E. Katoen, E.M.P.H. de Jong‐Vrancken, C. Starren‐Goessens, C.M.R. Haekens, and J.M.J. Lipsch‐Crijns for the inclusion of patients. We also thank H. van Eijk and J. Bastings for laboratory support.
Ubachs J., Ziemons J., Soons Z., Aarnoutse R., van Dijk D. P. J., Penders J., van Helvoort A., Smidt M. L., Kruitwagen R. F. P. M., Baade‐Corpelijn L., Olde Damink S. W. M., and Rensen S. S. (2021) Gut microbiota and short‐chain fatty acid alterations in cachectic cancer patients, Journal of Cachexia, Sarcopenia and Muscle, 12, 2007–2021, 10.1002/jcsm.12804
References
- 1. Argiles JM, Busquets S, Stemmler B, López‐Soriano FJ. Cancer cachexia: understanding the molecular basis. Nat Rev Cancer 2014;14:754–762. [DOI] [PubMed] [Google Scholar]
- 2. von Haehling S, Anker SD. Prevalence, incidence and clinical impact of cachexia: facts and numbers—update 2014. J Cachexia Sarcopenia Muscle 2014;5:261–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fearon K, Arends J, Baracos V. Understanding the mechanisms and treatment options in cancer cachexia. Nat Rev Clin Oncol 2013;10:90–99. [DOI] [PubMed] [Google Scholar]
- 4. Tremaroli V, Backhed F. Functional interactions between the gut microbiota and host metabolism. Nature 2012;489:242–249. [DOI] [PubMed] [Google Scholar]
- 5. Bindels LB, Neyrinck AM, Loumaye A, Catry E, Walgrave H, Cherbuy C, et al. Increased gut permeability in cancer cachexia: mechanisms and clinical relevance. Oncotarget 2018;9:18224–18238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bindels LB, Thissen J‐P. Nutrition in cancer patients with cachexia: a role for the gut microbiota? Clin Nutr Exp 2016;6:74–82. [Google Scholar]
- 7. Ziemons J, Smidt M, Damink SO, Rensen SS. Gut microbiota and metabolic aspects of cancer cachexia. Best Pract Res Clin Endocrinol Metab 2021;35:101508. [DOI] [PubMed] [Google Scholar]
- 8. Bindels LB, Neyrinck AM, Claus SP, Le Roy CI, Grangette C, Pot B, et al. Synbiotic approach restores intestinal homeostasis and prolongs survival in leukaemic mice with cachexia. ISME J 2016;10:1456–1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bindels LB, Beck R, Schakman O, Martin JC, De Backer F, Sohet FM, et al. Restoring specific lactobacilli levels decreases inflammation and muscle atrophy markers in an acute leukemia mouse model. PLoS ONE 2012;7:e37971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Varian BJ, Goureshetti S, Poutahidis T, Lakritz JR, Levkovich T, Kwok C, et al. Beneficial bacteria inhibit cachexia. Oncotarget 2016;7:11803–11816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Faber J, Vos P, Kegler D, Van Norren K, Argiles JM, Laviano A, et al. Beneficial immune modulatory effects of a specific nutritional combination in a murine model for cancer cachexia. Br J Cancer 2008;99:2029–2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Oliphant K, Allen‐Vercoe E. Macronutrient metabolism by the human gut microbiome: major fermentation by‐products and their impact on host health. Microbiome 2019;7:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Morrison DJ, Preston T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes 2016;7:189–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. van der Beek CM, Dejong CH, Troost FJ, Masclee AA, Lenaerts K. Role of short‐chain fatty acids in colonic inflammation, carcinogenesis, and mucosal protection and healing. Nutr Rev 2017;75:286–305. [DOI] [PubMed] [Google Scholar]
- 15. Martin L, Senesse P, Gioulbasanis I, Antoun S, Bozzetti F, Deans C, et al. Diagnostic criteria for the classification of cancer‐associated weight loss. J Clin Oncol 2015;33:90–99. [DOI] [PubMed] [Google Scholar]
- 16. Costea PI, Zeller G, Sunagawa S, Pelletier E, Alberti A, Levenez F, et al. Towards standards for human fecal sample processing in metagenomic studies. Nat Biotechnol 2017;35:1069–1076. [DOI] [PubMed] [Google Scholar]
- 17. Caporaso JG, Lauber CL, Walters WA, Berg‐Lyons D, Huntley J, Fierer N, et al. Ultra‐high‐throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME JIsme j 2012;6:1621–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bakker‐Zierikzee AM, Alles MS, Knol J, Kok FJ, Tolboom JJ, Bindels JG. Effects of infant formula containing a mixture of galacto‐ and fructo‐oligosaccharides or viable Bifidobacterium animalis on the intestinal microflora during the first 4 months of life. Br J Nutr 2005;94:783–790. [DOI] [PubMed] [Google Scholar]
- 19. Zhao G, Nyman M, Jönsson JA. Rapid determination of short‐chain fatty acids in colonic contents and faeces of humans and rats by acidified water‐extraction and direct‐injection gas chromatography. Biomed Chromatogr 2006;20:674–682. [DOI] [PubMed] [Google Scholar]
- 20. Thiel R, Blaut M. An improved method for the automated enumeration of fluorescently labelled bacteria in human faeces. J Microbiol Methods 2005;61:369–379. [DOI] [PubMed] [Google Scholar]
- 21. van der Sluijs Veer G, van den Hoven B, Russel MG, van den Bergh FA. Time‐resolved fluorimetric immunoassay of calprotectin: technical and clinical aspects in diagnosis of inflammatory bowel diseases. Clin Chem Lab Med 2006;44:292–298. [DOI] [PubMed] [Google Scholar]
- 22. Lagkouvardos I, Joseph D, Kapfhammer M, Giritli S, Horn M, Haller D, et al. IMNGS: a comprehensive open resource of processed 16S rRNA microbial profiles for ecology and diversity studies. Sci Rep 2016;6:33721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. RC Team . R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. 2020. Available from: https://www.R‐project.org/ [Google Scholar]
- 24. de Wert LA, Rensen SS, Soons Z, Poeze M, Bouvy ND, Penders J. The cutaneous microbiome in hospitalized patients with pressure ulcers. Sci Rep 2020;10:5963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA‐seq data with DESeq2. Genome Biol 2014;15:550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lagkouvardos I, Fischer S, Kumar N, Clavel T. Rhea: a transparent and modular R pipeline for microbial profiling based on 16S rRNA gene amplicons. PeerJ 2017;5:e2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Peterson BG, Carl P. PerformanceAnalytics: econometric tools for performance and risk analysis. R package version 2.0.4. 2020. Available from: https://CRAN.R‐project.org/package=PerformanceAnalytics
- 28. McGregor K, Labbe A, Greenwood CMT. MDiNE: a model to estimate differential co‐occurrence networks in microbiome studies. Bioinformatics 2020;36:1840–1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ogle DH, Wheeler P, Dinno A. FSA: fisheries stock analysis. R package version 0.8.32. 2021. Available from: https://github.com/droglenc/FSA
- 30. Pötgens SA, Thibaut MM, Joudiou N, Sboarina M, Neyrinck AM, Cani PD, et al. Multi‐compartment metabolomics and metagenomics reveal major hepatic and intestinal disturbances in cancer cachectic mice. J Cachexia Sarcopenia Muscle 2021;12:456–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lozupone CA, Stombaugh JI, Gordon JI, Jansson JK, Knight R. Diversity, stability and resilience of the human gut microbiota. Nature 2012;489:220–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pötgens SA, Brossel H, Sboarina M, Catry E, Cani PD, Neyrinck AM, et al. Klebsiella oxytoca expands in cancer cachexia and acts as a gut pathobiont contributing to intestinal dysfunction. Sci Rep 2018;8:12321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Morgan XC, Tickle TL, Sokol H, Gevers D, Devaney KL, Ward DV, et al. Dysfunction of the intestinal microbiome in inflammatory bowel disease and treatment. Genome Biol 2012;13:R79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Menezes‐Garcia Z, Do Nascimento Arifa RD, Acúrcio L, Brito CB, Gouvea JO, Lima RL, et al. Colonization by Enterobacteriaceae is crucial for acute inflammatory responses in murine small intestine via regulation of corticosterone production. Gut Microbes 2020;11:1531–1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Borgo F, Riva A, Benetti A, Casiraghi MC, Bertelli S, Garbossa S, et al. Microbiota in anorexia nervosa: the triangle between bacterial species, metabolites and psychological tests. PLoS ONE 2017;12:e0179739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bach Knudsen KE, Lærke HN, Hedemann MS, Nielsen TS, Ingerslev AK, Gundelund Nielsen DS, et al. Impact of diet‐modulated butyrate production on intestinal barrier function and inflammation. Nutrients 2018;10:1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hernández MAG, Canfora EE, Jocken JW, Blaak EE. The short‐chain fatty acid acetate in body weight control and insulin sensitivity. Nutrients 2019;11:1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Burri E, Beglinger C. The use of fecal calprotectin as a biomarker in gastrointestinal disease. Expert Rev Gastroenterol Hepatol 2014;8:197–210. [DOI] [PubMed] [Google Scholar]
- 39. Pin F, Barreto R, Couch ME, Bonetto A, O'Connell TM. Cachexia induced by cancer and chemotherapy yield distinct perturbations to energy metabolism. J Cachexia Sarcopenia Muscle 2019;10:140–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Aarnoutse R, Ziemons J, Penders J, Rensen SS, de Vos‐Geelen J, Smidt ML. The clinical link between human intestinal microbiota and systemic cancer therapy. Int J Mol Sci 2019;20:4145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ng CD. Errors in body mass index from self‐reported data by sex and across waves of Add Health. Ann Epidemiol 2019;39:21–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kim EY, Lee HY, Kim YS, Park I, Ahn HK, Cho EK, et al. Prognostic significance of cachexia score assessed by CT in male patients with small cell lung cancer. Eur J Cancer Care 2018;27:e12695. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Correlations between SCFA and inflammatory parameters.
Table S2a. Disease stages per cancer type. Information on tumor stages was retrieved from medical records (if available*) and classified as local disease, lymph node involvement, or metastatic disease.
Table S2b. Disease stages in cachectic and non‐cachectic cancer patients. Information on tumor stages was retrieved from medical records (if available*) and classified as local disease, lymph node involvement or metastatic disease.
Figure S1. Dendrograms show no clustering of samples based on cachexia status (upper panel) or BMI (lower panel). We observed high inter‐individual heterogeneity.
Figure S2. Genera abundance across different grades of BMI‐adjusted weight loss. This categorization into grade 0 – grade 4 was suggested in Martin et al. (2015). Diagnostic criteria for the classification of cancer‐associated weight loss. Journal Clinical Oncology, 2015. 33(1): p. 90–9. Each grade was associated with significantly different survival, whereby a gradient of decreasing survival was observed with increasing weight loss and decreasing BMI (grade 0 = longest survival; grade 4 = shortest survival).
Figure S3. Log2 abundance of genera which were differentially abundant between cachectic cancer patients, non‐cachectic cancer patients, and healthy controls, analyzed for pancreatic cancer and lung cancer patients separately. A: The likelihood ratio test (LRT) indicated significant differential abundance of Enterococcus (p < 0.001), Lactobacillus (p < 0.01), unknown Enterobacteriaceae (p < 0.001) and Veillonella (p < 0.001) between cachectic and non‐cachectic pancreatic cancer patients and healthy controls. Differences which were significantly different according to the Wald test (α = 0.05) are marked with asterisks. B: LRT indicated that only Lactobacillus (p < 0.001) showed differential abundance in cachectic and non‐cachectic lung cancer patients and healthy controls. Differences which were significantly different according to the Wald test (α = 0.05) are marked with asterisks.
Figure S4. SCFA levels in patients with pancreatic cancer or lung cancer. A: Acetate levels were significantly lower in cachectic pancreatic cancer patients (N = 16) compared to non‐cachectic pancreatic cancer patients (N = 5). Total SCFA and butyrate concentrations tended to be reduced in cachectic patients. B: Levels of all SCFA were similar in cachectic (N = 5) and non‐cachectic lung cancer patients (N = 25).
Figure S5. Fecal levels of the BCFA iso‐butyrate and iso‐valerate in cachectic cancer patients (N = 30), non‐cachectic cancer patients (N = 64), as well as in healthy controls (N = 71). Levels of iso‐butyrate (p = 0.608) and iso‐valerate (p = 0.543) were similar in all groups.


