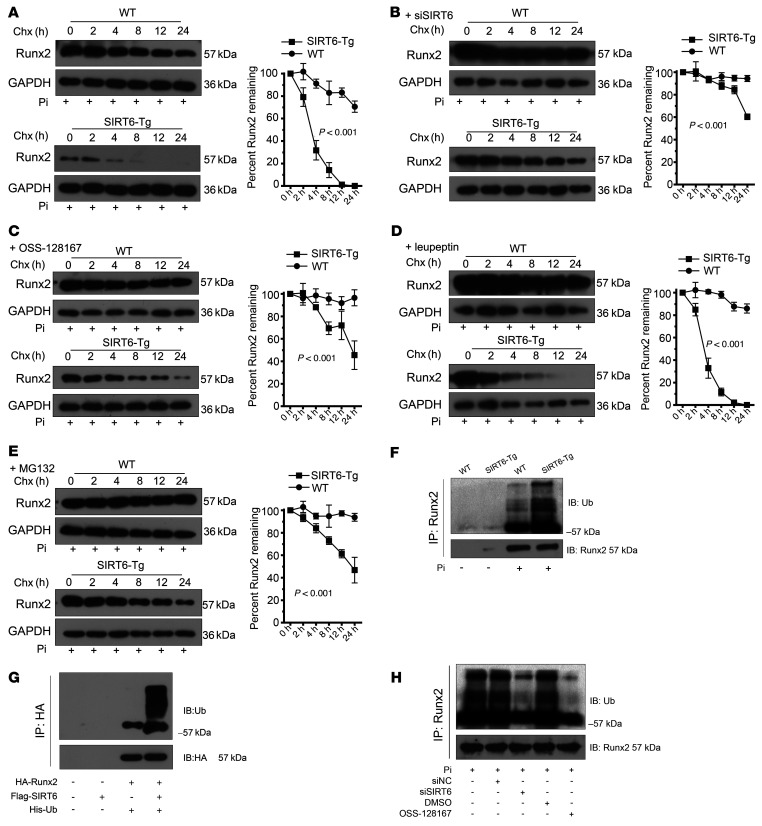
Figure 5. SIRT6 promotes Runx2 degradation via the ubiquitin-proteasome system.
(A) WT and SIRT6-Tg VSMCs were treated with Pi (3.0 mM) for 7 days and incubated with the protein translation inhibitor CHX (0.2 mM) for the indicated times before harvest, followed by immunoblotting with the anti-Runx2 antibody and anti-GAPDH anti-body. The curve shows the stability of Runx2 protein. (B and C) SIRT6 was decreased in primary VSMCs via siRNA (B) or specific inhibitor (C) together with Pi (3.0 mM) incubation for 7 days. The protein translation inhibitor CHX (0.2 mM) was added for indicated times before harvest, followed by immunoblotting with the anti-Runx2 antibody and anti-GAPDH antibody. The curve shows the stability of Runx2 protein. (D and E) SIRT6-Tg VSMCs were incubated with Pi (3.0 mM) together with the leupeptin (1.5 μM) (D) or MG132 (10 μM) (E) for 7 days, and then the protein translation inhibitor CHX (0.2 mM) was added for the indicated times before harvest, followed by immunoblotting with the anti-Runx2 antibody and anti-GAPDH antibody. The curve shows the stability of Runx2 protein. (F) WT and SIRT6-Tg VSMC lysates were immunoprecipitated with anti-Runx2 antibody and immunoblotted with anti-ubiquitin (anti-Ub) antibody. (G) HEK-293T cells were transfected with His-Ub together with HA-Runx2 plasmid, flag-SIRT6 plasmid, or both. The anti-HA IP was followed by Western blot with anti-Ub antibody and anti-HA antibody. (H) SIRT6-Tg VSMCs were pretransfected with siSIRT6 or siNC together with Pi (3.0 mM) for 7 days, and OSS-128167 or DMSO were incubated with Pi (3.0 mM) for 7 days. The cell lysates were immunoprecipitated with anti-Runx2 antibody and immunoblotted with anti-Ub antibody and anti-Runx2 antibody. Statistical significance was assessed using 2-way ANOVA (A–E). All the above experimental processing was duplicated 3 times.