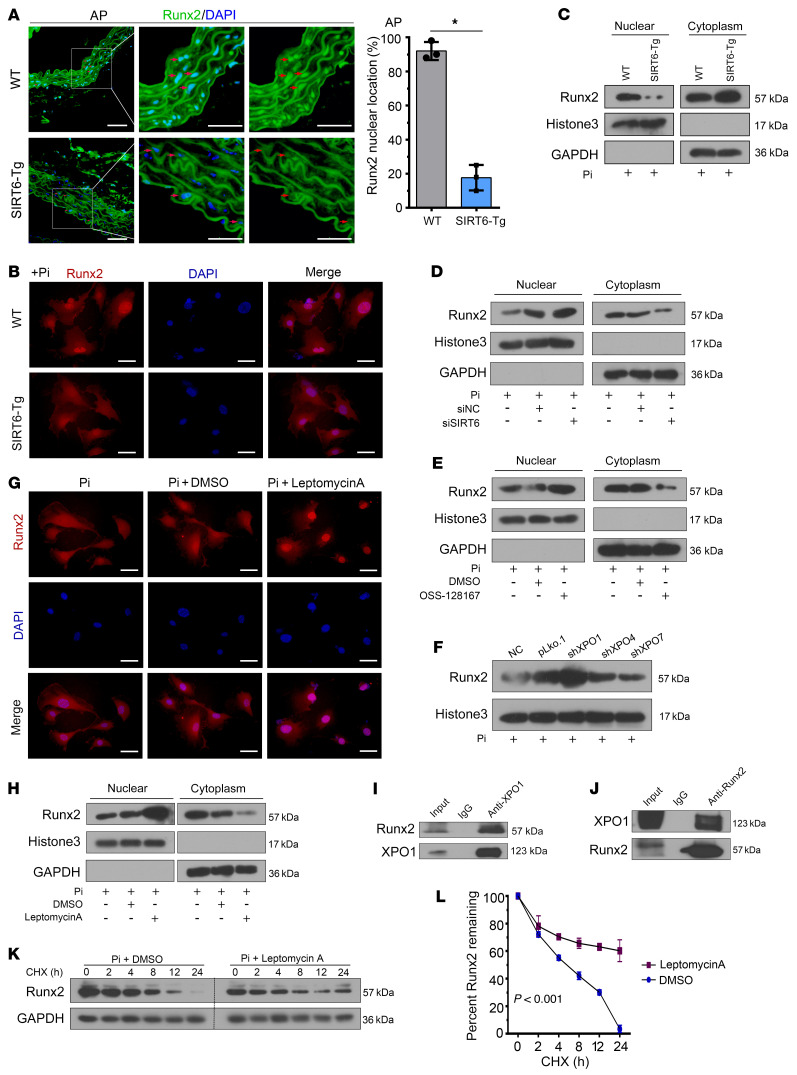
Figure 6. SIRT6 mediates Runx2 nuclear export depending on XPO1.
(A) Runx2 IF staining was performed in abdominal arteries. Scale bar: 50 μm. Statistical significance was assessed using 2-tailed t tests, *P < 0.05. (B) VSMCs were incubated with Pi for 7 days. IF staining was performed for Runx2. Scale bars: 50 μm. (C) VSMCs were incubated with Pi for 7 days. Cells were harvested and immunoblotted for the indicated proteins. (D) SIRT6-Tg VSMCs were incubated with Pi for 7 days after posttransfection of siSIRT6. Cells were harvested and immunoblotted for the indicated proteins. (E) SIRT6-Tg VSMCs were incubated with Pi together with nicotinamide for 7 days. Cells were harvested and immunoblotted for the indicated proteins. (F) SIRT6-Tg VSMCs were transfected with shRNA targeting XPO1, XPO4, XPO7, or their vector control, and then incubated with Pi for 7 days after transfection. Nuclear extracts were immunoblotted for Runx2. (G and H) SIRT6-Tg VSMCs were incubated with Pi together with Leptomycin A (0.5 nM) for 7 days. Cells were harvested and immunoblotted for the indicated proteins (G). IF staining was performed for Runx2. Scale bars: 50 μm (H). (I) Anti-XPO1 IP followed by Western blot with anti-Runx2 or anti-XPO1 antibody in SIRT6-Tg VSMCs after treatment with Pi for 7 days. Anti-rabbit IgG IP was used as negative control. (J) Anti-Runx2 IP in SIRT6-Tg VSMCs after treatment with Pi for 7 days. Western blot was carried out with anti-XPO1 or anti-Runx2 antibody. Anti-mouse IgG IP was used as negative control. (K) SIRT6-Tg VSMCs were incubated with Pi together with Leptomycin A for 7 days, and then CHX (0.2 mM) was added for the indicated times before harvest, followed by immunoblotting for the indicated proteins. (L) Curve shows the stability of Runx2 and was assessed using 2-way ANOVA. Pi treatment is 3.0 mM. All the above experimental processing was duplicated 3 times.