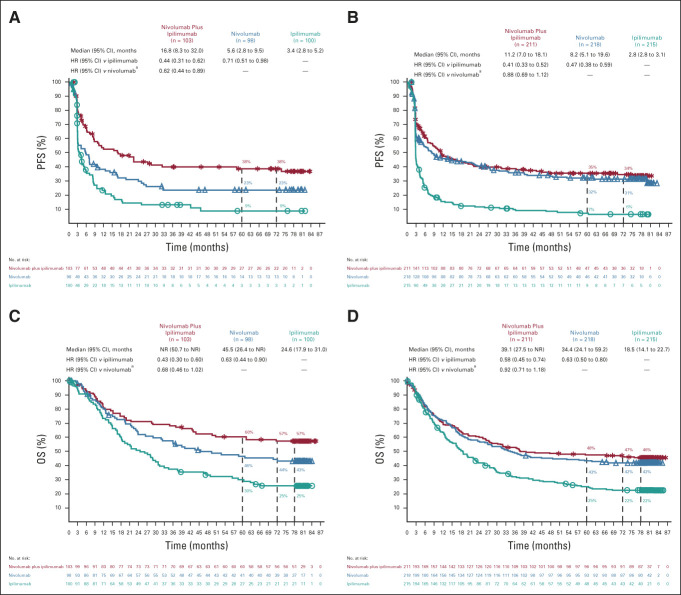
FIG 4.
PFS in patients with (A) BRAF-mutant or (B) BRAF–wild-type tumors and OS in patients with (C) BRAF-mutant or (D) BRAF–wild-type tumors. Patients were followed for a minimum of 77 months. In the nivolumab plus ipilimumab, nivolumab, and ipilimumab groups, 314, 316, and 315 patients had BRAF mutational status results, respectively. aDescriptive analysis. HR, hazard ratio; NR, not reached; OS, overall survival; PFS, progression-free survival.