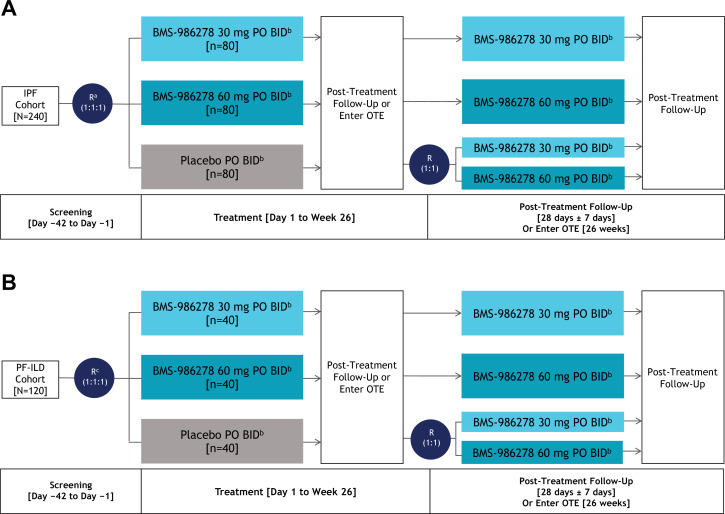
Figure 1.
Study Design: (A) IPF and (B) PF-ILD Cohorts. aRandomisation will be stratified (1) by country (Japan vs rest of world); and (2) according to concomitant use of approved IPF therapy (pirfenidone vs nintedanib vs none). bPatients receiving 30 mg or 60 mg BMS-986278 two times per day or placebo two times per day in the main study or OTE who meet low BP criteria may have their dosage reduced to 10 mg BMS-986278 two times per day (or matching placebo for 10 mg two times per day in main study). cRandomisation will be stratified by (1) UIP pattern (typical or probable UIP vs inconsistent with UIP) of lung injury on either centrally read HRCT, surgical lung biopsy, or cryobiopsy; and (2) according to background therapy (immunosuppression (azathioprine, mycophenolate mofetil, mycophenolic acid and/or tacrolimus) vs antifibrotic agents (pirfenidone or nintedanib) vs none). BID, two times per day; BP, blood pressure; HRCT, high-resolution CT; IPF, idiopathic pulmonary fibrosis; OTE, optional active-treatment extension; PF-ILD, progressive fibrotic interstitial lung disease; PO, per os (by mouth); R, randomisation; UIP, usual interstitial pneumonia.