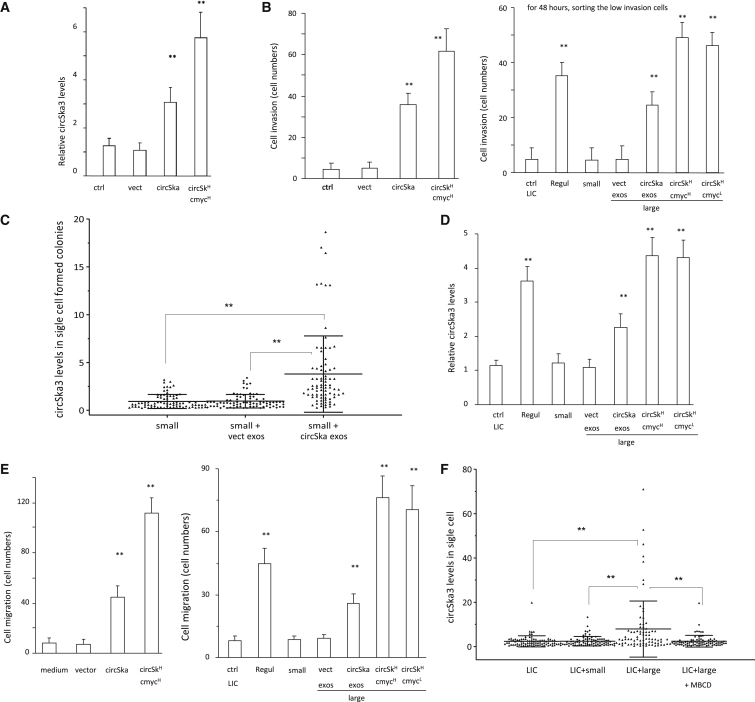
Figure 8.
Transfer of circSKA3 potentiates cell invasion
(A) Small-colony cells (n = 4) were cultured with a basal medium, vectored packed exosomes, and circSKA3 packed exosomes (100 ug/mL) for 3 days, and the medium was changed every 8 h. Small-colony cells showed increased circSKA3 levels after circSKA3-packed exosome treatment. However, they still showed lower circSKA3 levels than large-colony cells (circSKA3H/c-mycH group; n = 4). (B) Left, circSKA3-packed exosome treatment increased small-colony cell invasion. Right, LICs showed a promoted invasion ability after being co-cultured with large-colony cells (n = 4). (C) Small-colony cells were co-cultured without or with exosomes purified from the vector- or circSKA3-transfected cells. Treatment with exosomes from the circSKA3 cells increased circSKA3 levels . (D) 1,000 LICs were co-cultured with 200 LICs, regular culture cells, small-colony cells, and large-colony cells (circSKA3H/c-mycH and circSKA3H/c-mycL). All of these 200 cultured cells were stably transfected with GFP. After 48 h, the no-GFP LICs were sorted by flow cytometry, and 200 cells were processed to real-time PCR (n = 4). LICs showed increased circSKA3 after being co-cultured with large-colony cells. (E) Left, a co-culture with cells expressing high levels of circSKA3 increased the quantity of circSKA3 tumor cell migration. Right, LICs displayed promoted cell migration after being co-cultured with large-colony cells (n = 4). (F) LICs were co-cultured with small-colony cells, large-colony cells (circSKA3H/c-mycH), and LICs co-cultured with large-colony cells and MβCD. Treatment with large-colony cells increased levels of circSKA3 and resulted in a similar pattern as in the regular cells, which could be blocked by MβCD. ∗∗ p<0.01; Error bars, SD