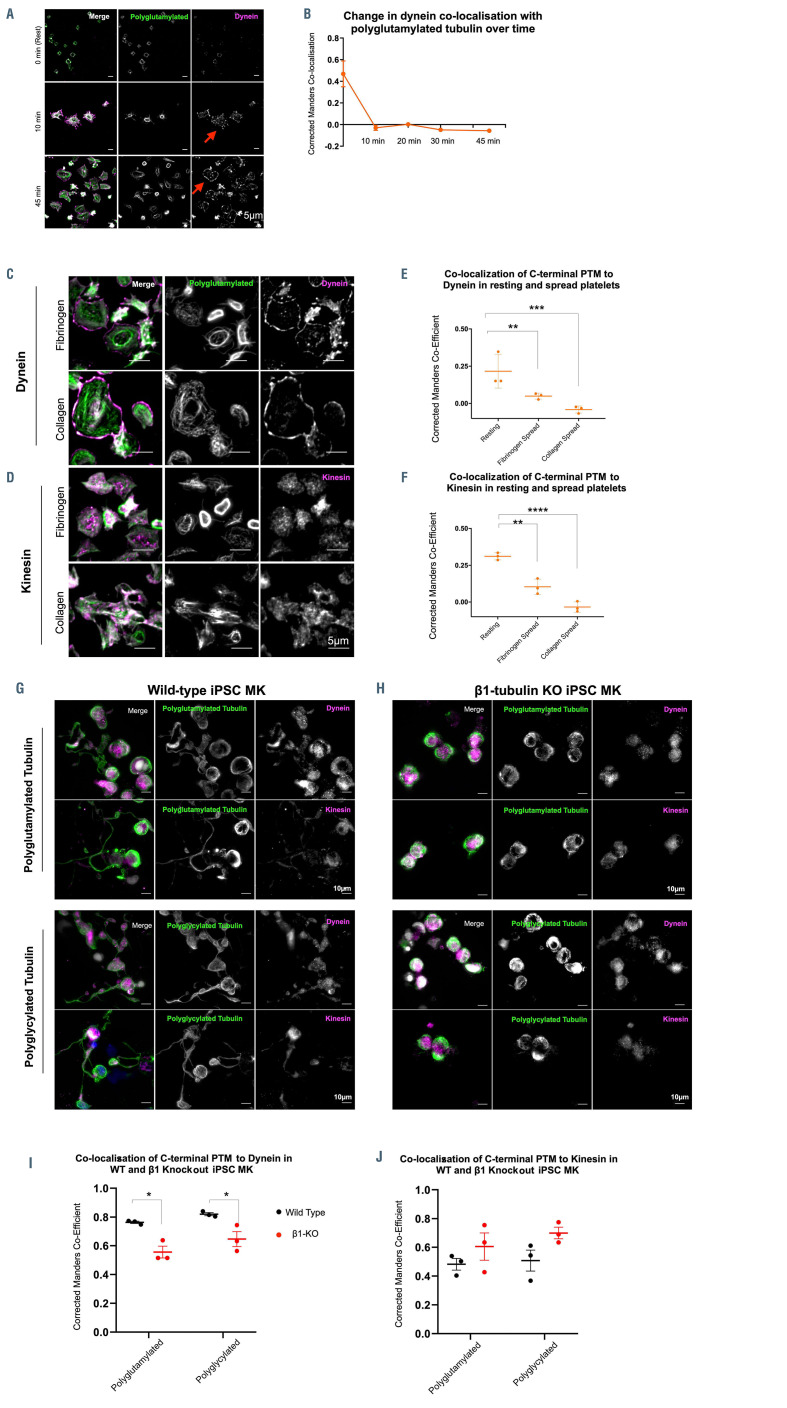
Figure 5.
Polyglutamylation regulates spatial distribution of motor proteins in platelets and megakarycytes. (A and B) Quantification of the co-localization of dynein, as measured by the corrected Manders coefficient, with polyglutamylated tubulin over time shows a marked decrease in co-localization between these polymodified residues and the motor over time. (C) In fibrinogen and collagen spread cells, dynein is observed on the periphery of the spread cells, (D) while kinesin-1 is evident as a diffuse punctate stain. (E) Dynein co-localization with polyglutamylated residues decreases dramatically on platelet spreading in both fibrinogen and collagen (**P=0.0082 and ***P=0.0004 respectively). (F) A loss of co-localization with polyglutamylated tubulin is evident in fibrinogen and collagen spread cells (**P=0.0017, ****P<0.0001 respectively). (G) Immunofluorescence staining of induced pluripotent stem cell megacaryocytes (iPSC-MK) for polymodified tubulin, dynein, and kinesin-1 show the distribution of both motors along the length of the proplatelet shaft in wild-type (WT) cells. (H and I) In b1-tubulin knockout (KO) iPSCMK, no proplatelet extensions are formed and a significant reduction in the co-localization of dynein to polyglutamlated residues is observed (*P=0.0166 and *P=0.0293 respectively), (J) with no significant change in the co-localization of kinesin-1 with polyglycylated tubulin. (n=3, standard deviation. Two-Way ANOVA with multiple comparisons.)