Extended Data Fig. 3. Comparison of the inactive β1-AR receptor with the activated β1-AR in complex with Gi.
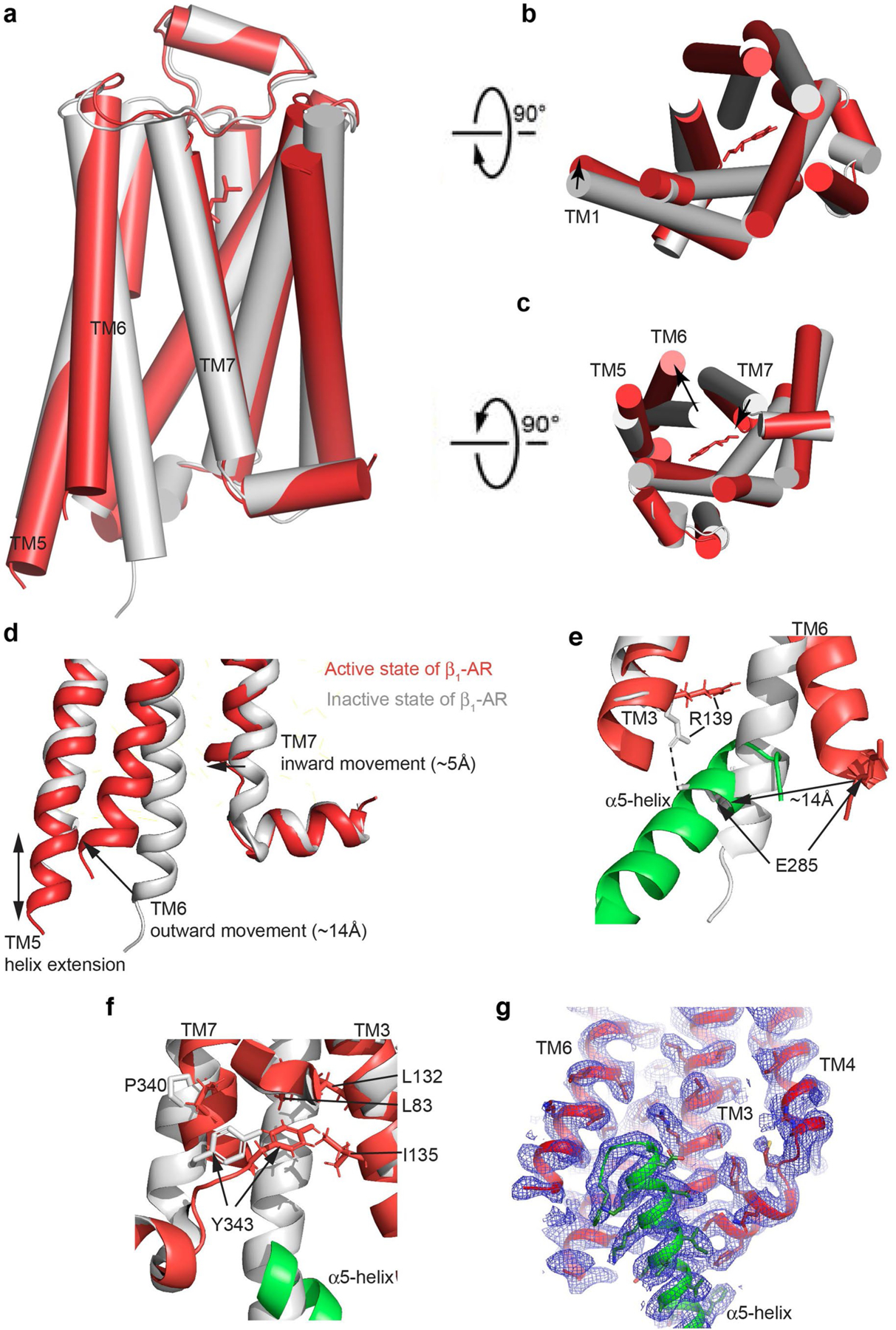
(a-c) Side (left), extracellular (top right) and cytoplasmic (bottom right) views of the inactive β1-AR (grey)(PDB code 4GPO) compared to the activated β1-AR in complex with Gi. (d) Structural comparison of the Gi α5-helix binding region to show the major conformational changes of β1-AR. (e) The ionic lock between Arg139 and Glu285 is broken in the active state. (f) Tyr343 packs against Leu132 and Ile135 in the active state. (g) Cryo-EM density map of the Gi α5-helix binding region (contoured at 1.2 σ level).
