Extended Data Fig. 8. Conformational changes of the GDP-binding pocket of Gαs after β1-AR interaction.
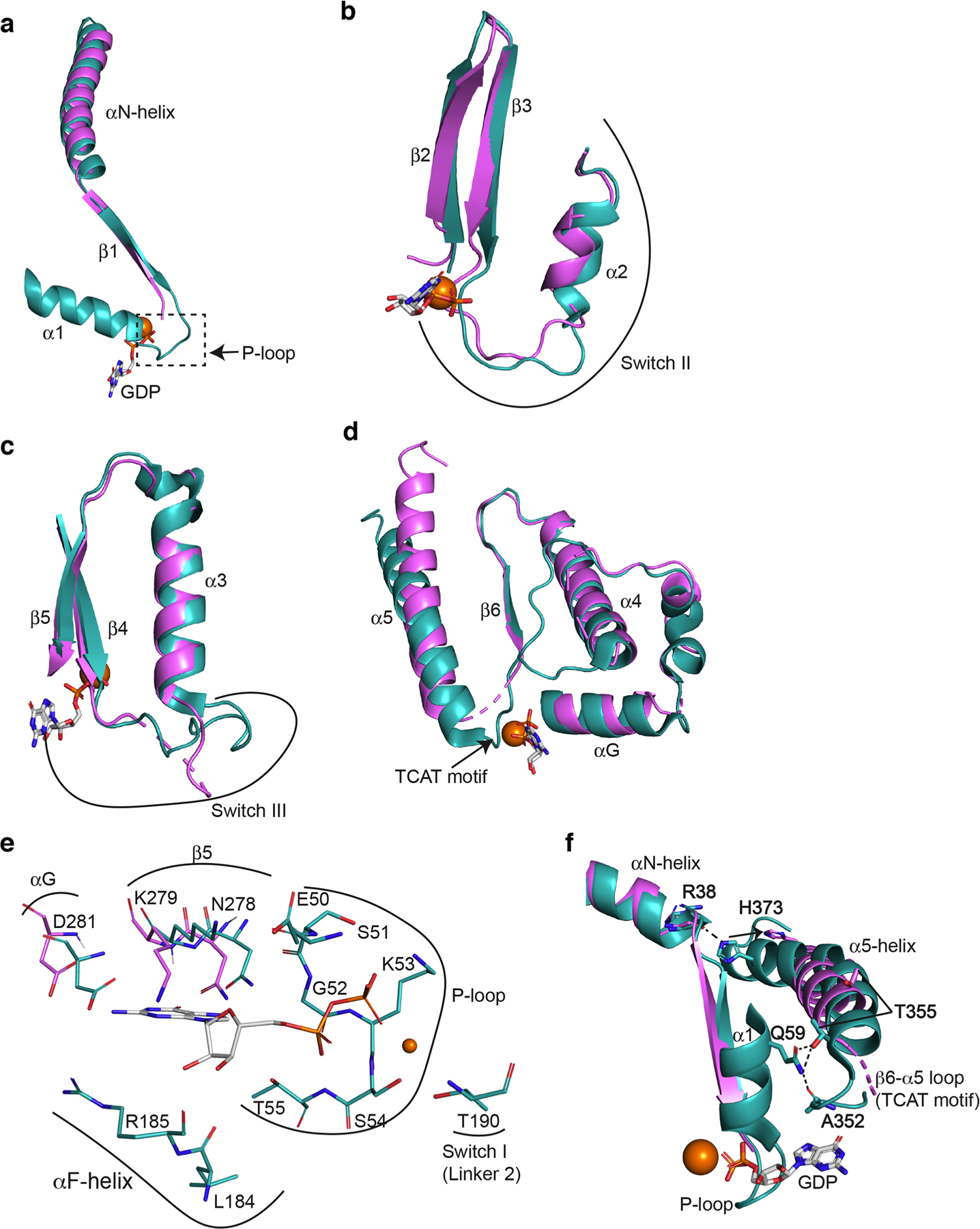
(a) Comparison of the β1 strand, α1-helix and the β1-α1 loop of the Ras-like domains from β1-AR-Gs (in violet) and from GαsGβ1Gγ2 (PDB: 6EG8; in teal) when the Ras-like domains are superimposed. (b) Comparison of Switch II region from β1-AR-Gs and from GαsGβ1Gγ2. (c) Comparison of Switch III region from β1-AR-Gs and from GαsGβ1Gγ2(d) Comparison of the regions from αG to α5-helix from β-1AR-Gs and from GαsGβ1Gγ2. (e) Comparison of all GDP-interacting residues of the Ras-like domains from β1-AR-Gs and from GαsGβ1Gγ2.(f) Disruptions of intra-molecular interactions of Gαs during Gs activation by β1-AR. An interaction between the sidechain of His373 in the α5-helix and the backbone of Arg38 in the αN-helix is broken. An interacting network involving the sidechain of Gln59 in the αl-helix, the backbone carbonyl of Ala352 in the β6-α5 loop, and the sidechain of Thr355 in the α5-helix is disrupted.
