Fig. 2. Structural basis of the activation of Gi by β1-AR.
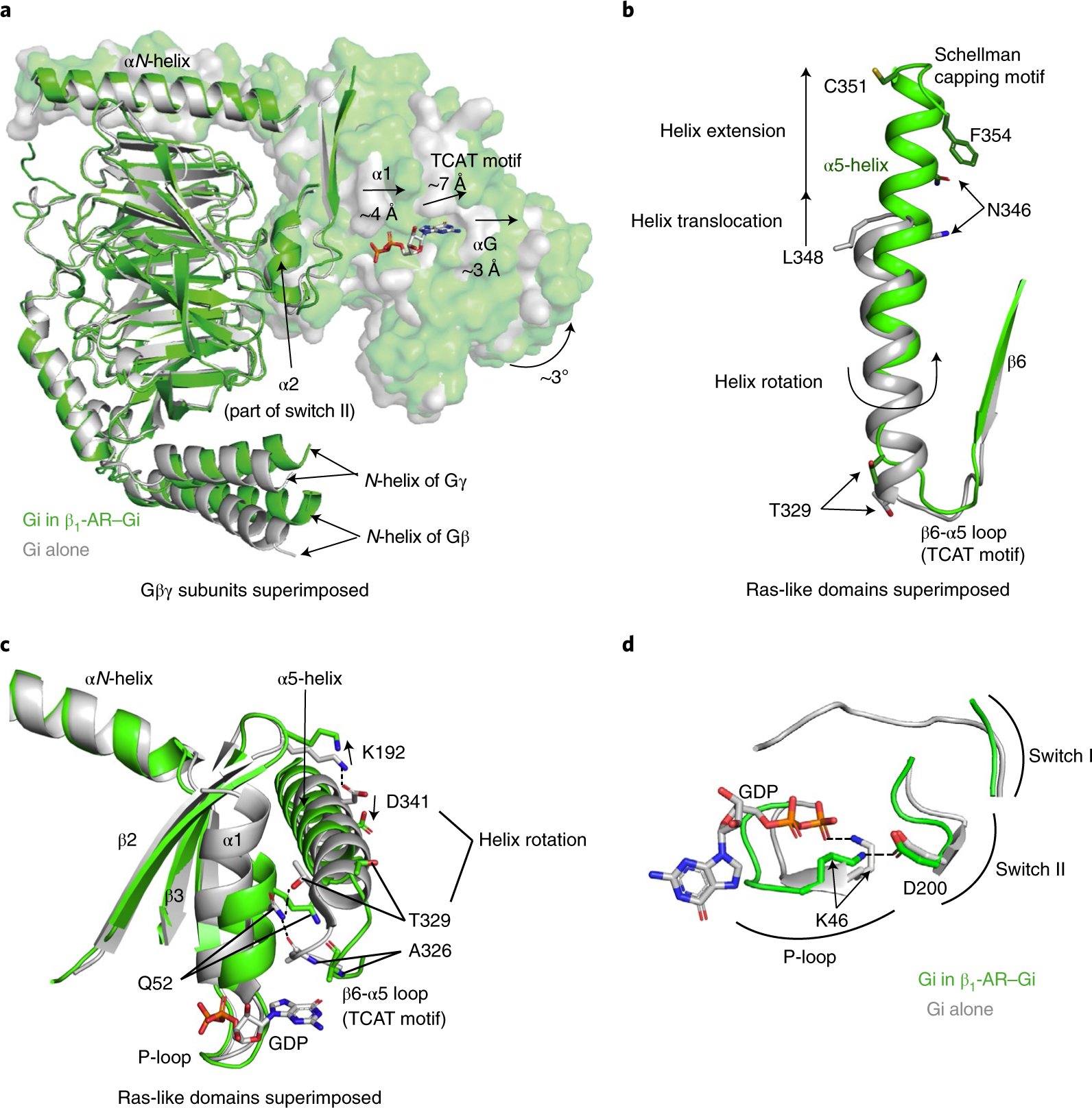
a, Structural changes of the GDP-binding pocket of Gαi in the complex of β1-AR–Gi (in green) when compared with the inactive Gαi(GDP)Gβγ trimer (in gray), when Gβγ subunits are superimposed. b, Structural differences in the α5-helices of the β1-AR–Gi complex (in green) and in the inactive GαiGβγ heterotrimer (in gray), when the Ras-like domains are superimposed. c, Disruptions of the α5- and α1-helix interactions of Gαi during Gi activation by β1-AR. An ionic interaction between the side chain of Asp341 in the α5-helix and the side chain of Lys192 in the β2-β3 loop is broken. An interacting network involving the side chain of Gln52 in the α1-helix, the backbone carbonyl of Ala326 in the β6-α5 loop, and the side chain of Thr329 in the α5-helix is disrupted. d, A newly formed interaction between Lys46 in the α1-helix (part of the P-loop) and Asp200 in β3 (part of Switch II) during or after GDP release.
