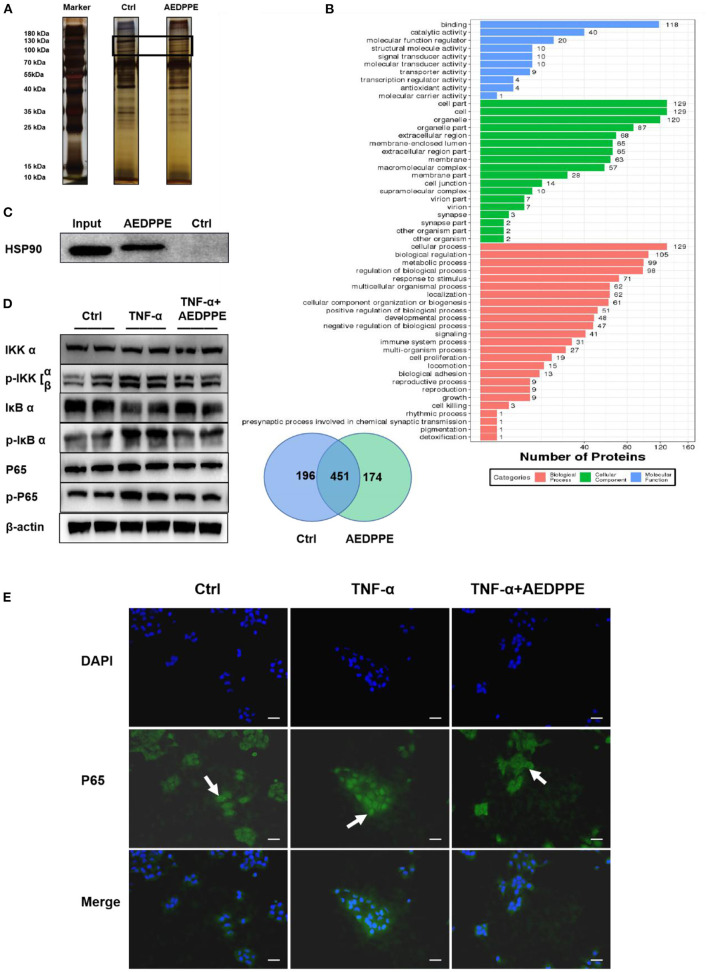
Figure 4.
Improvement in HTR-8/SVneo cell function by AEDPPE may involve the NF-κB signaling pathway by binding to HSP 90β. (A) Pull-down assay showed that more proteins specifically bound to biotin-AEDPPE at the markers shown compared to those in the control group. (B) Gels with differences between the AEDPPE and control groups were analyzed using mass spectrometry and subjected to GO analysis, and we noted protein differences between the two groups. (C) AEDPPE bound specifically to HSP 90β in vitro. (D) TNF-α strongly activated the NF-κB pathway, resulting in increased p-IKK expression, catabolic depletion of IκB α, and increased p-IκB α–ultimately leading to increased expression levels of p-P65. Activation of the NF-κB signaling pathway was inhibited when AEDPPE was co-stimulated with TNF-α compared to TNF-α alone. (E) The distribution of P65 (green) in HTR-8/SVneo cells was localized by immunofluorescence. Only unstimulated phosphorylated P65 translocated into the nucleus (blue), although stimulation by TNF-α caused a large amount of P65 to translocate into the nucleus; and P65 in the nucleus was reduced when co-stimulated with TNF-α and AEDPPE (scale bar = 100 μm). IL, interleukin; PlGF, placental growth factor; sFlt-1, soluble FMS-like tyrosine kinase 1; TNF-α, tumor-necrosis factor alpha.