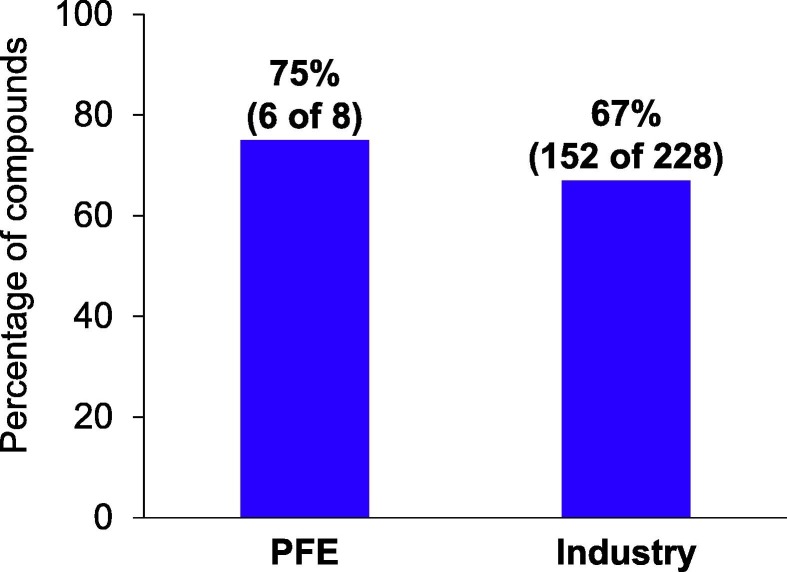
Figure 2.
Percentage of new molecular entities (NMEs) with at least one regulatory expedited designation, from 2016 to 2020. Regulatory designations include Breakthrough Therapy, Priority Review, Fast Track, and Accelerated Approval. This was calculated using a 5-year rolling average and excluded designations from the US Food and Drug Administration (FDA) Center for Biologics Evaluation and Research (CBER) to be consistent. As a result, Comirnaty vaccine for COVID-19 was excluded from this analysis. Data from Pfizer internal tracking and FDA Center for Drug Evaluation and Research.