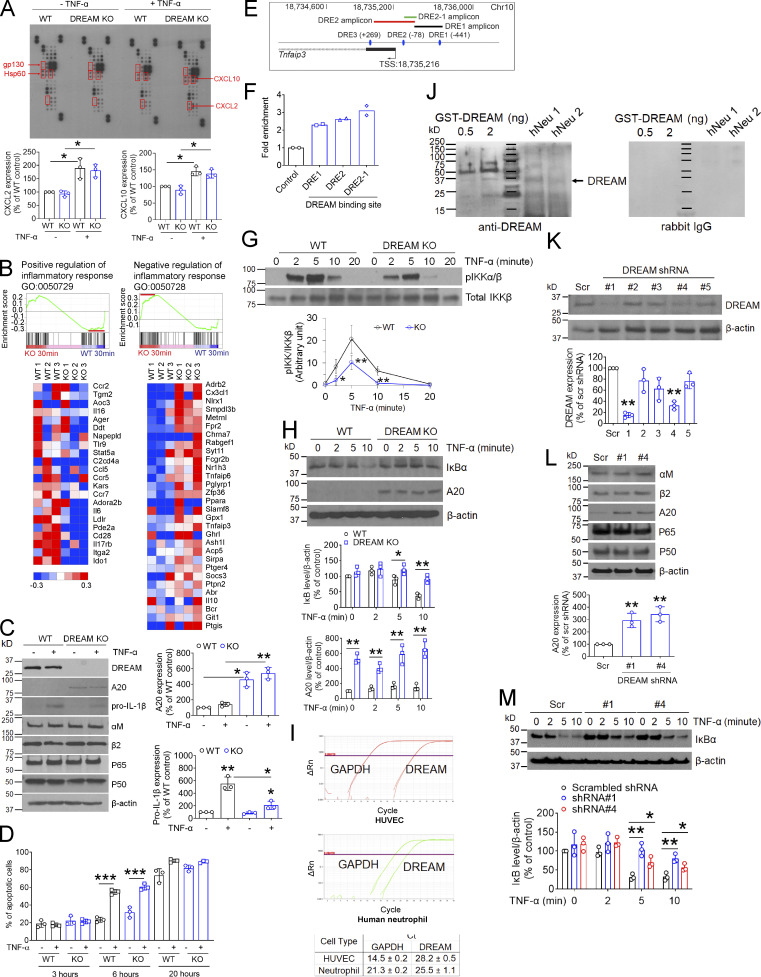
Figure 3.
Neutrophil DREAM down-regulates A20 expression and positively regulates NF-κB activity. (A) Chemokine microarray with the lysate of WT and DREAM KO neutrophils treated without or with TNF-α for 3 h. HSP60 and gp130 were used as loading controls. Densitometric analysis of CXCL2 and CXCL10 expression after normalization to HSP60 expression. (B) WT and DREAM KO neutrophils were treated with TNF-α for 30 min and subjected to RNA-seq. GSEA shows the gene expression altered by deletion of neutrophil DREAM. Heat map of genes selected from pro- and anti-inflammatory response pathways in WT and KO neutrophils (n = 3). Red, up-regulation; blue, down-regulation. (C) WT and DREAM KO neutrophils were treated with or without 5 ng/ml TNF-α for 3 h. Equal amounts (50 µg) of proteins in cell lysates were immunoblotted. Representative blots (n = 3). Densitometric analysis of A20 and pro–IL-1β expression. (D) WT and DREAM KO neutrophils were treated with 20 ng/ml TNF-α for 3, 6, and 20 h and used for flow cytometry with FITC-conjugated annexin V. (E and F) Mouse neutrophils were used for ChIP analysis. (E) The DREAM-binding sites (DRE sites) and transcriptional start sites (TSSs) in Tnfaip3. Three DRE amplicons using several different primers are shown. DRE1 amplicon: 18,735,396–18,735,671 (275 bp), DRE2 amplicon: 18,734,944–18,735,414 (469 bp), and DRE2-1 amplicon: 18,735,305–18,735,422 (117 bp). (F) DREAM enrichment was calculated by the fold increase in Gfi1b (control). Data represent the mean of two experiments. (G) WT and DREAM KO neutrophils were incubated with 5 ng/ml TNF-α for 0–20 min. Lysates were immunoblotted with antibodies against pIKKα/β or total IKKβ and subjected to densitometric analysis. (H) WT and DREAM KO neutrophils were incubated with 5 ng/ml TNF-α for 0–10 min and subjected to immunoblotting with antibodies against IκBα, A20, or β-actin, and densitometric analysis. (I) RT-qPCR of HUVECs and neutrophils. (J) GST-tagged DREAM (0.5 and 2 ng) expressed in Escherichia coli and lysates of neutrophils isolated from two donors (hNeu 1 and hNeu 2) were used for immunoblotting with rabbit anti-DREAM antibodies. Representative blots (n = 3). (K–L) HL-60 cells treated with scrambled (Scr) or DREAM shRNAs were differentiated for 5–7 d and subjected to immunoblotting with the indicated antibodies and densitometric analysis. (M) dHL-60 cells were treated with 5 ng/ml TNF-α for 0–10 min and subjected to immunoblotting with anti-IκBα antibodies and densitometric analysis. Data represent the mean ± SD (n = 3 or 4). *, P < 0.05; **, P < 0.01; and ***, P < 0.001 versus WT neutrophils or control shRNA after Student’s t test (D and G–M) or ANOVA and Tukey’s test (C).