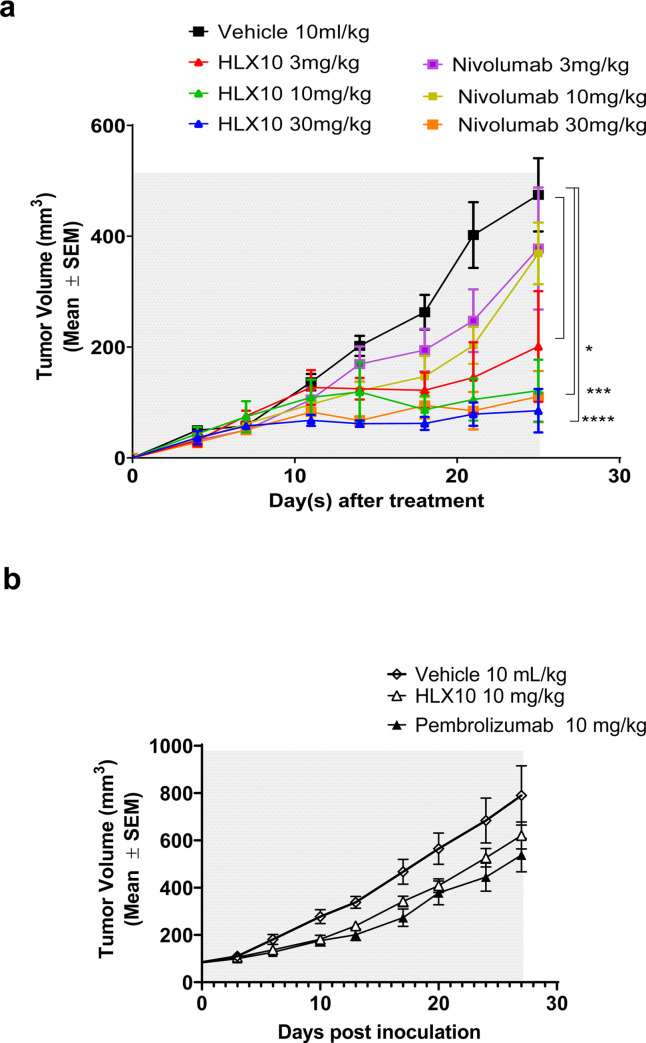
Fig 7. HLX10 in vivo antitumor activity compared to that of Nivolumab and Pembrolizumab.
(a) NOD/SCID mice engrafted with NCI-H292/hPBMC co-mixture (same as Fig 5C and 5D), and treated with vehicle, HLX10 or Nivolumab at the indicated doses; n = 8 mice/group. (b) CD34+ humanized NSG mice engrafted with MDA-MB-231-HM model and treated with vehicle, HLX10 or pembrolizumab (Keytruda®), intraperitoneally. Antibodies were administered at a dose level of 10 mg/kg on day 0 and the remaining doses were maintained at 5 mg/kg for a Q7D × 5 schedule (gray area). The mice in vehicle group were intraperitoneally injected with PBS following the same Q7D × 5 schedule. TGI are represented as the means ± SEM.