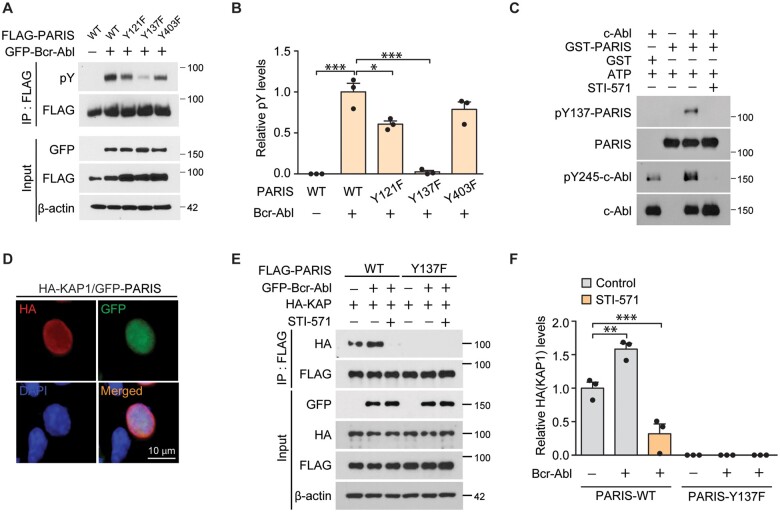
Figure 1.
Activated c-Abl tyrosine-phosphorylates PARIS. (A) Representative immunoblots of PARIS tyrosine phosphorylation using a phospho-tyrosine specific antibody (pY) on anti-FLAG immunoprecipitation samples from SH-SY5Y cells transiently transfected (48 h) with N-terminal FLAG-tagged wild-type (WT) or mutants with substitutions of phenylalanine for tyrosine residues that are potentially phosphorylated (Y121F, Y137F and Y403F) with or without GFP-Bcr-Abl (constitutively active form). β-Actin serves as an internal loading control. (B) Quantification of relative tyrosine phosphorylation of anti-FLAG immunoprecipitated PARIS wild-type and mutants (n = 3 per group). (C) In vitro kinase assay demonstrating GST-tagged recombinant PARIS (GST-PARIS) phosphorylation at Y137 by recombinant c-Abl kinase assessed by immunoblotting using an anti-pY137-PARIS antibody. STI (10 µM) was used to block c-Abl, and c-Abl activity was monitored with an anti-pY245-c-Abl antibody. Similar results were obtained from two independent experiments. (D) Representative immunofluorescence images depicting the nuclear expression of GFP-PARIS and HA-KAP in SH-SY5Y cells transfected with HA-KAP and GFP-PARIS (48 h) using antibodies against the HA or GFP tags. Scale bar = 10 μm. (E) Immunoblots depicting the association between HA-KAP and FLAG-PARIS in anti-FLAG immunoprecipitation samples from SH-SY5Y cells transfected with the indicated combination of HA-KAP, FLAG-tagged PARIS wild-type or a Y137F phosphorylation-deficient PARIS mutant, and GFP-Bcr-Abl with or without c-Abl inhibitor STI-571 treatment (10 µM). β-Actin serves as an internal loading control. (F) Quantification of HA-KAP levels in the anti-FLAG co-immunoprecipitates from the indicated experimental groups (n = 3 per group). Data are expressed as mean ± SEM. Statistical ANOVA test followed by Tukey’s post hoc analysis or unpaired two-tailed Student’s t-test. *P < 0.05, **P < 0.01 and ***P < 0.001.