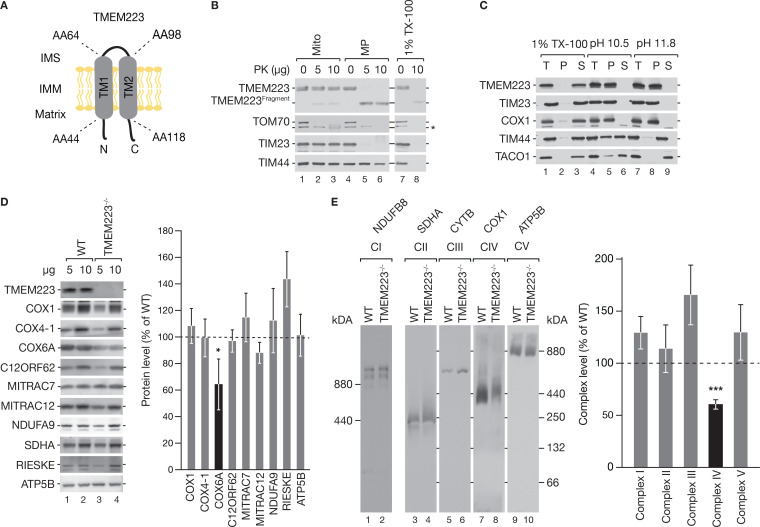
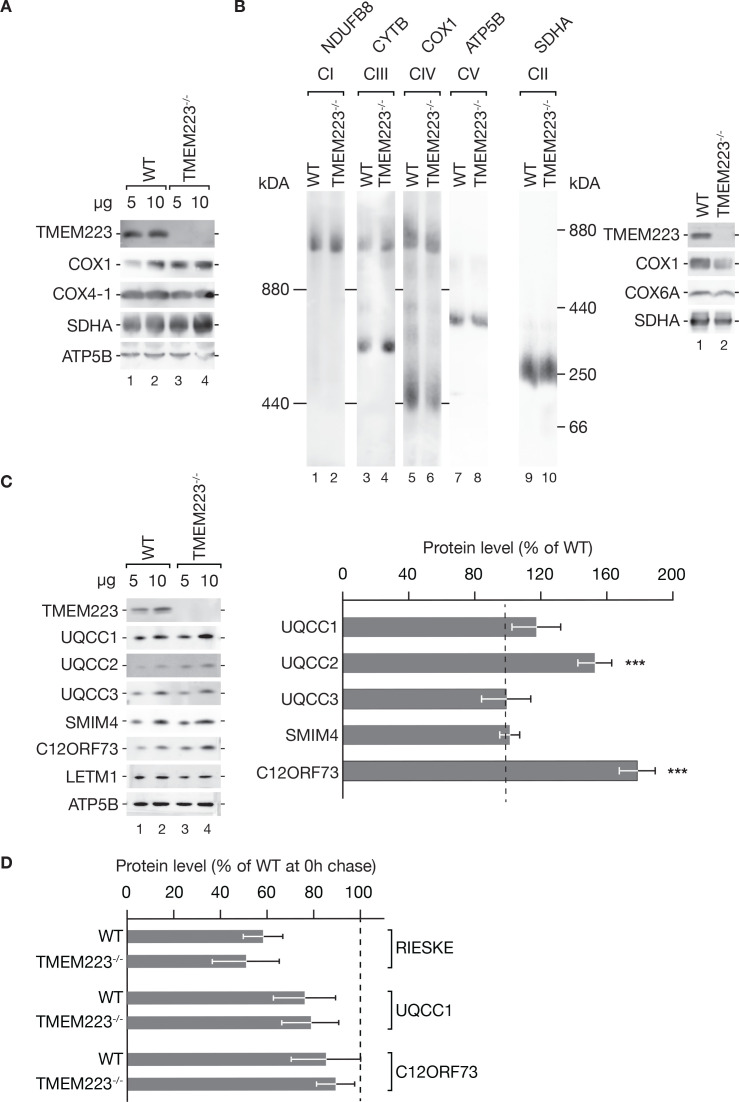
Figure 2. TMEM223 is a mitochondrial membrane protein.
(A) Membrane topology of TMEM223. The predicted transmembrane spans (TM1 and TM2) with corresponding amino acids (aa) are indicated. IMS: intermembrane space; IMM: inner mitochondrial membrane. (B) and (C) Submitochondrial localization of TMEM223. Wild-type (WT) mitochondria were treated with Proteinase K (PK) under iso-osmotic (Mito), hyper-osmotic conditions (swelling, MP), or solubilized with Triton X-100 (TX-100) (B). The unspecific band is marked with an asterisk. Mitochondrial proteins were extracted in sodium carbonate containing buffer at different pH (total, T; pellet, P; soluble fraction, S) (C). (D) Protein steady-state levels in TMEM223−/− cells. Mitochondrial lysates from WT and TMEM223−/− cells were analyzed by western blotting using indicated antibodies and protein amounts were quantified using ImageQuant software (mean ± SEM, n=3). (E) Isolated mitochondria from WT and TMEM223−/− cells were solubilized in DDM-containing buffer, separated on 2.5–10% (Complex I) or 4–13% (Complexes II–V) BN-PAGE and analyzed by western blotting. OXPHOS complexes were detected with indicated antibodies and amounts quantified using ImageQuant software(mean ± SEM, n=3).