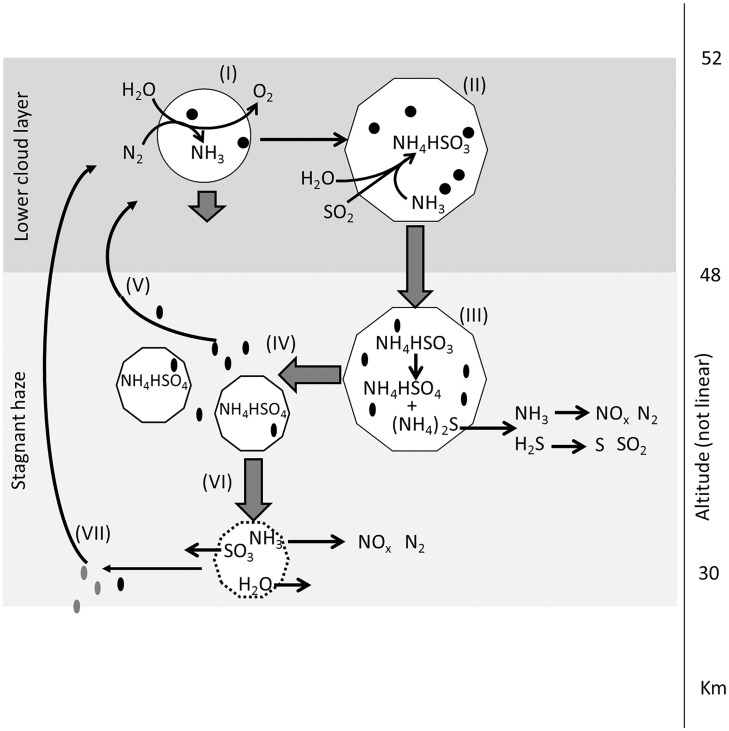
Fig. 2.
Ammonia cycle in the atmosphere of Venus. See SI Appendix, section 10 for details. I: NH3 is produced locally in the clouds from atmospheric N2 and H2O (Table 1) by metabolically active microorganisms (black dots) inhabiting cloud droplets (white circle). II: The production of NH3 in the droplet raises the droplet pH to −1 to 1 (from −11 on the Hammett acidity scale) by trapping the SO2 and H2O in the droplet as ammonium hydrogen sulfite (NH4HSO3). The production of sulfite salts in the droplet leads to the formation of a large, semisolid (and hence nonspherical) Mode 3 particle (white decagon). III: The Mode 3 particle settles out of the clouds where ammonium sulfite disproportionates to ammonium sulfate and ammonium sulfide; the latter decomposes to H2S and NH3, which, in turn, undergo photochemical reactions to a variety of products. IV: Disproportionation and gas release break up the Mode 3 particles into smaller haze particles and microorganism spores (black ovals), some of which return to the cloud layer (V). VI: The ammonium sulfate particles fall farther below the cloud decks, where ammonium sulfate decomposes to SO3, NH3, and H2O. VII: Spores released at this stage may be unviable (gray ovals), but any surviving could also be eventually transported back to the clouds.