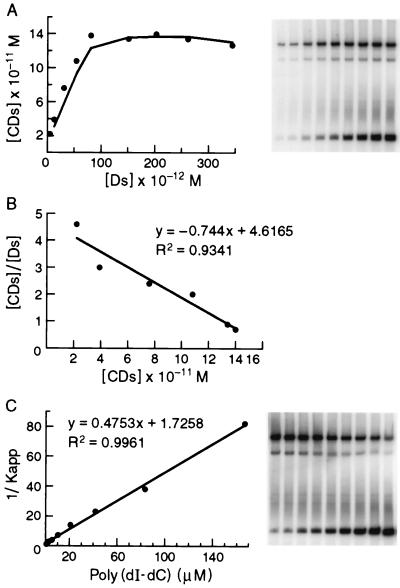
FIG. 3.
Determination of an equilibrium binding constant for CgCbf1p binding to a C. glabrata centromere DNA fragment. (A) Saturation binding curve as determined from experiments using increasing amounts of radiolabeled CgCEN DNA and a constant amount of affinity-purified CgCbf1p in the presence of nonspecific poly(dI-dC) DNA. The concentration of CgCbf1p that bound to CgCEN, [CDs], is plotted versus that of free CgCEN, [Ds]. (B) Scatchard plot of the data in panel A (see Materials and Methods for details). The slope of the line equals Kapp, and the x intercept equals C0, the number of binding sites in the reaction. (C) Determination of the equilibrium constant for nonspecific binding (Kn) to correct Kapp for the contribution made by binding of CgCbf1p to nonspecific DNA. Constant amounts of radiolabeled CgCEN and affinity-purified CgCbf1p were incubated with increasing amounts of poly(dI-dC) DNA. The inverse of Kapp (determined from equation 3, see Materials and Methods) is plotted versus the concentration of nonspecific DNA, [Dn0]. Both Kn and Ks can be determined. The y intercept equals 1/Ks, and the slope of the line is equal to Kn/Ks. Thus, Kn is equal to the slope divided by the y intercept.