Figure 11.
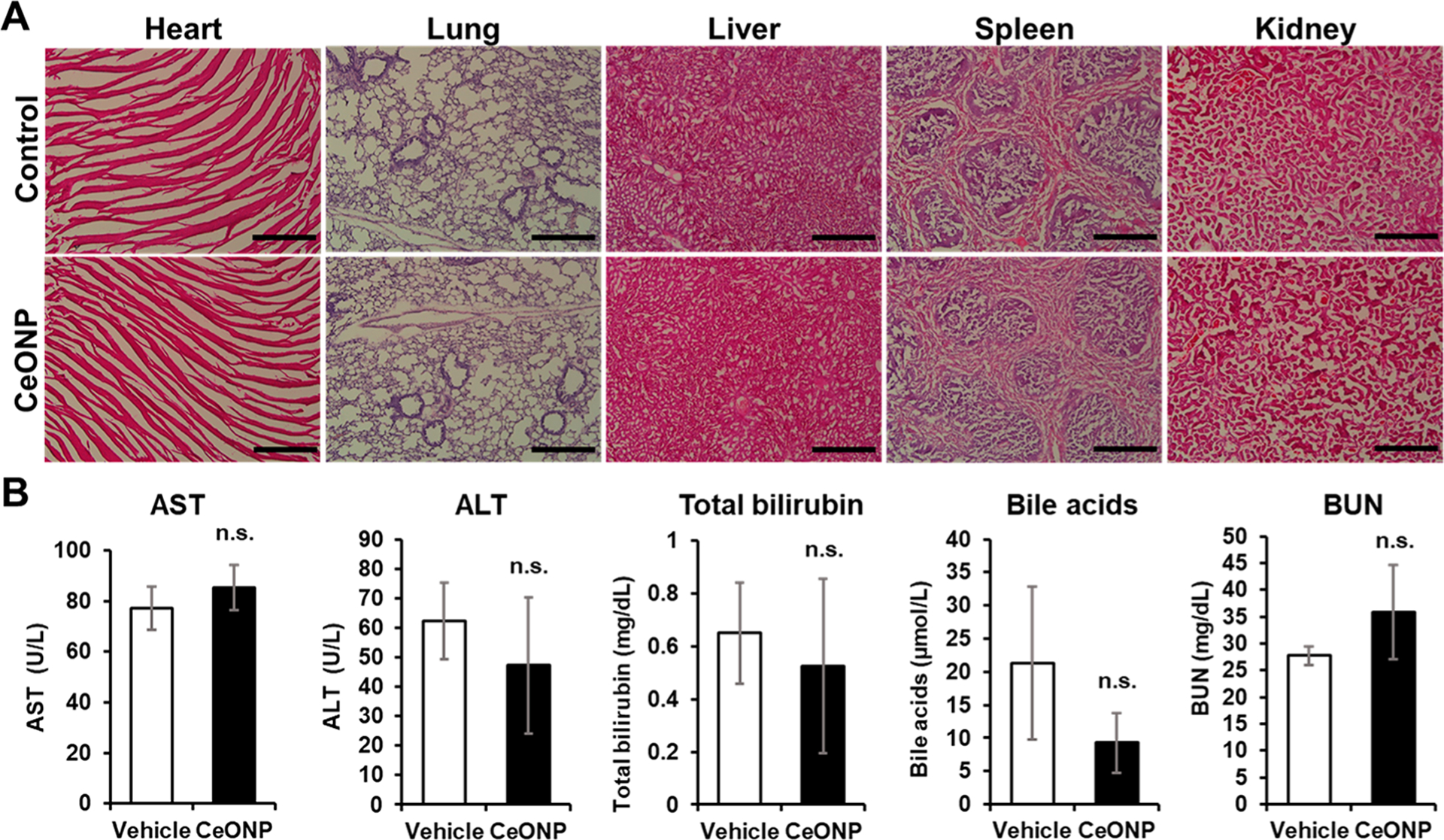
In vivo toxicity of CeONP from histological and biomarker analysis. (A) Micrographs of H&E-stained major organs (heart, lung, liver, spleen, and kidney) and (B) serum biomarker levels of liver and kidney functions from mice 24 h after injection with the vehicle (control) or CeONP at 100 mg kg−1. Scale bar = 50 μm (mean ± SEM; n = 4 per group; n.s. = not significant relative to the vehicle).
