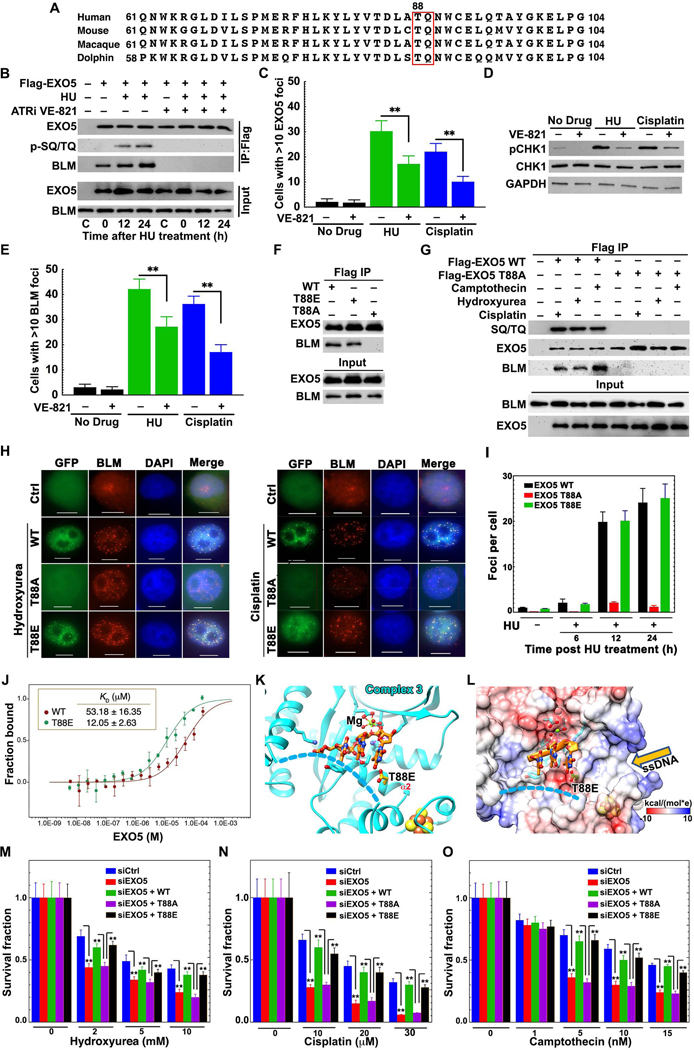
Figure 6. ATR Phosphorylation of EXO5 T88 Promotes BLM Interaction and Damage Foci Formation.
(A) Multiple EXO5 sequence alignment shows invariant -TQ- ATR recognition motif. (B) Detection of EXO5 phosphorylation and BLM interaction by western blot of Flag IP with anti-SQ/TQ antibody and BLM antibody in cells treated with or without HU or ATR inhibitor VE-821. (C, E) Quantitative analysis of EXO5 (GFP-EXO5 expression) and BLM foci formation in HeLa cells treated with HU or cisplatin with or without VE-821. (D) Western blotting with phospho-CHK1 antibody detects ATR inhibition by VE-821. (F) Interaction of HA-Flag-EXO5 variants with BLM by IP without drug treatment. (G) Phosphorylation of EXO5 variants and BLM interaction after drug treatment. Anti-HA antibody detected HA-Flag-EXO5 expression in (B), (F) and (G). (H) Co-localization of drug-induced DNA damage foci for EXO5 variants with BLM. Scale bar: 10 μm. (I) Quantitative foci analysis of EXO5 variants with HU treatment. (J) Binding affinity between BLMcat and EXO5 (WT and T88E) measured by MST. Atto-647-labeled BLMcat was titrated with EXO5 variants in triplicates. MST fluorescence changes were normalized as fraction bound. (K) EXO5-T88E-DNA Complex 3 (DNA, orange sticks) bound to Mg2+. (L) Electrostatic surface view of Complex 3 showing local negative charge near DNA binding path (disordered crossover-helix, blue dash-lines). (M-O) Clonogenic survival assays for EXO5-depleted cells with WT or EXO5 T88 mutant’s complementation after HU, cisplatin, or CPT treatment. For foci, 50 cells were analyzed for each experiment. Foci and cell survival data are averaged from three independent experiments (** p<0.01). See Figure S6.