Fig. 2.
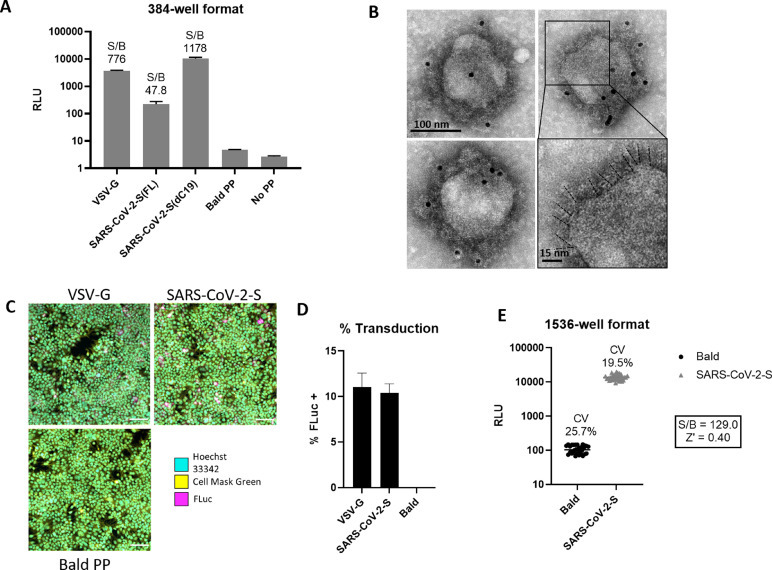
Characterization and optimization of SARS-CoV-2-S PP entry assay. (a) PP entry test in HEK293-ACE2 cells in 384-well format. Average relative luminescence unit (RLU) for bald PP (no glycoprotein) was used for signal-to-basal (S/B) calculations. Error bars represent standard deviations (SD). (b) EM of SARS-CoV-2-S(ΔC19) PP with immunogold labeling for SARS-CoV-2 spike. Bottom right shows enlarged area with putative spikes highlighted (dashed lines). (c) Immunofluorescence staining of luciferase in PP transduced HEK293-ACE2 cells. (d) Quantitation of luciferase positive cells by immunofluorescence staining. Error bars depict SD values. (e) Miniaturization of PP entry assay in 1536-well format. Z’ factor was calculated as Z’ = 1- 3(SDBasal + SDControl)/(MeanBasal – MeanControl), where basal samples are wells treated with SARS-CoV-2-S PPs and control samples are wells treated with bald PPs.
