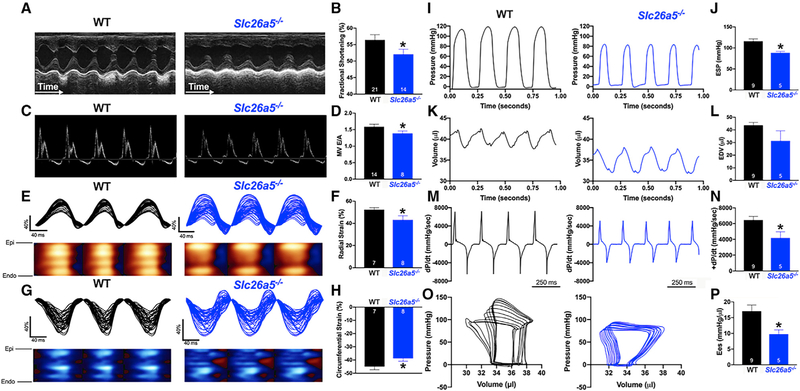
Figure 4. Echocardiographic recordings and hemodynamics monitoring demonstrate systolic and diastolic dysfunction in Slc26a5−/− mice.
(A) Representative M-mode echocardiography from WT and Slc26a5−/− mice.
(B) Summary data for fractional shortening (FS), illustrating a significant decrease in FS in knockout (KO) mice compared with that of WT.
(C) Pulsed Doppler velocity profile of inflow across the mitral valve (MV).
(D) Summary data showing a significant decrease in the E/A ratios in Slc26a5−/− compared with those of WT mice.
(E and G) Representative radial and circumferential strains in WT and Slc26a5−/− mice.
(F and H) Summary data for radial and circumferential strains showing a significant decrease in radial and circumferential strains in Slc26a5−/− mice compared with those of WT controls.
(I–P) Hemodynamic monitoring in WT and Slc26a5−/− mice showing left ventricular pressure (mmHg) (I and J), volume (μL) (K and L), and dP/dt (mmHg/s) (M and N). The pressure and volume have been calibrated. The volume calibration was performed using a cuvette (P/N 910–1049, Millar Inc.) filled with fresh heparinized 37°C mouse blood. (O) Pressure-volume relationship in WT and Slc26a5−/− mice during changes in preload. (P) Summary data for the end-systolic P-V relationship (Ees) (mmHg/μL) demonstrate reduced cardiac contractility in Slc26a5−/− mice. *p < 0.05.