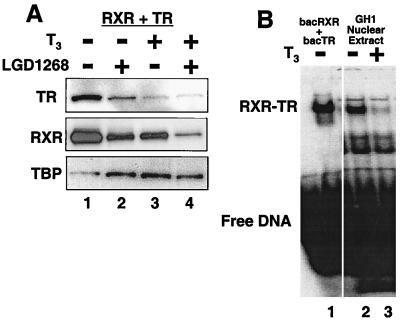
FIG. 9.
Both subunits of RXR-TR heterodimers are destabilized by ligand binding to TR. (A) CV1 cells were transfected with equal amounts of expression plasmids for hRXRα and hTRβ and incubated for 36 h in the absence (lane 1) or presence of 1.0 μM LGD1268 (RXR specific; lane 2), 100 nM T3 (TR specific; lane 3), or 1.0 μM LGD1268 plus 100 nM T3 (lane 4). After incubation with ligands, protein levels were examined by Western blotting with anti-TR antibodies, anti-RXR antibodies, and anti-TBP antibodies. (B) GH1 cells were cultured for 24 h in the absence (lane 2) or presence (lane 3) of 10 nM T3. Nuclear extracts were prepared and equal amounts of total protein were used to examine binding to a 32P-labeled probe derived from the palindromic TR element in the growth hormone gene. Lane 1 contains baculovirus-expressed RXR and TR.