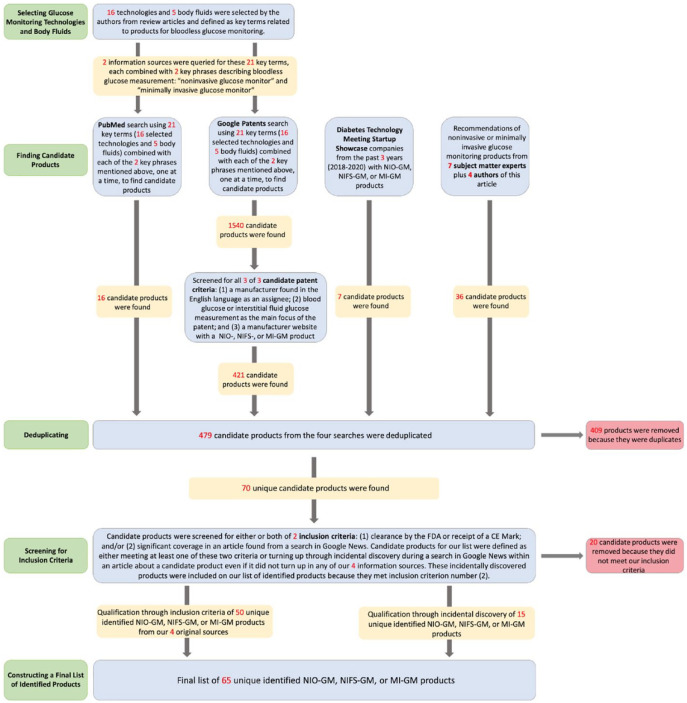
Figure 3.
A PRISMA diagram of how we performed our database reviews to create a list of identified NIO-GM, NIFS-GM, and MI-GM products.
Abbreviations: CE, Conformité Européenne; FDA, Food and Drug Administration; NIFS-GM, noninvasive fluid sampling glucose monitor; MI-GM, minimally invasive glucose monitor; NI-GM, noninvasive glucose monitor; NIO-GM, noninvasive optical glucose monitor; PRISMA, Preferred Reporting Items for Systematic Review and Meta-Analyses.