Table 5A.
Designs of NIO-GM, NIFS-GM, and MI-GM Identified Products with Regulatory Clearance (in Alphabetical Order Within Each Category of Invasiveness).
| Product and manufacturer | Degree of invasiveness | Product design |
|---|---|---|
| GlucoTrack; Integrity Applications Ltd | NIO-GM |
 293
293
|
| sugarBEAT; Nemaura Medical | NIFS-GM |
 326
326
|
| Cascade CGM System; Waveform | MI-GM |
 327
327
|
| Dexcom G6; Dexcom | MI-GM |
 328
328
|
| Eversense; Senseonics | Implanted MI-GM |
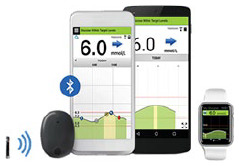 329
329
|
| FreeStyle Libre 14 day; Abbott Diabetes Care | MI-GM |
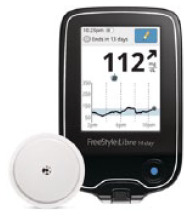 330
330
|
| FreeStyle Libre 2; Abbott Diabetes Care | MI-GM |
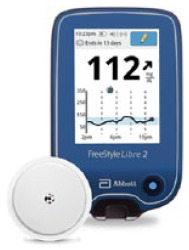 331
331
|
| FreeStyle Libre 3; Abbott Diabetes Care | MI-GM |
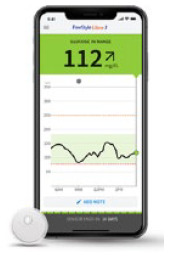 332
332
|
| Medtronic Guardian Connect (Powered by Medtronic Guardian Sensor 3); Medtronic MiniMed | MI-GM |
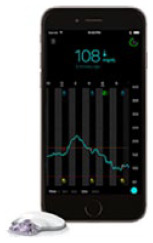 333
333
|
| PercuSense CGM; PercuSense | MI-GM |
 284
284
|
| Sencell; Lifecare AS | MI-GM |
 323
323
|
Abbreviations: MI-GM, minimally invasive glucose monitor; NIFS-GM, noninvasive fluid sensing glucose monitor; NIO-GM, noninvasive glucose monitor.
