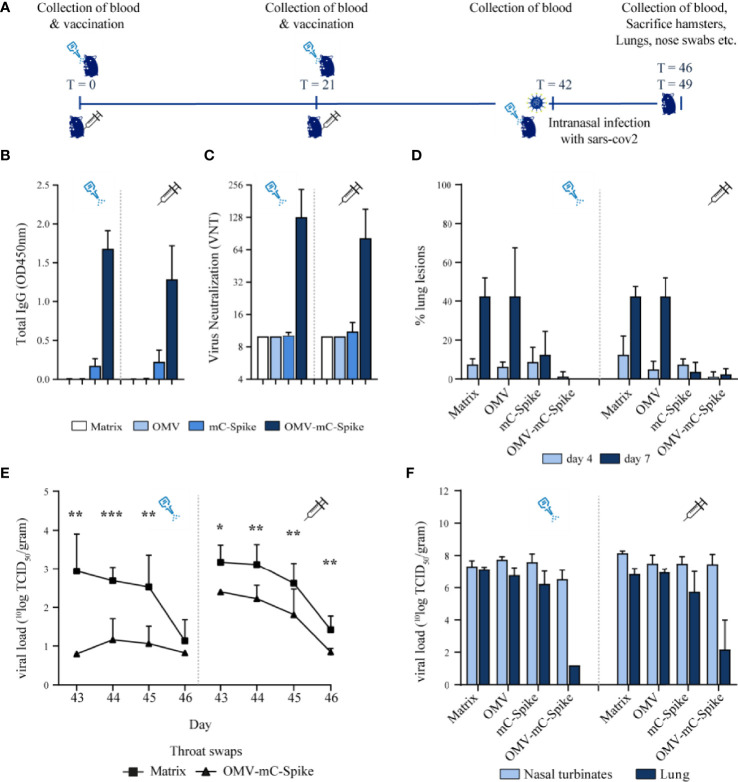
Figure 3.
Hamster challenge study. Animals were immunized intranasally or intramuscularly on day 0 and day 21 with 15 µg OMV, 15 µg spike or 15 µg OMV, and 15 µg Spike combined with mCRAMP. In the control group, animals were immunized with 10 mM Tris-3% sucrose, which is the OMV buffer. Sera were collected from all hamsters at experimental days 0, 21, 42, 46, and 49. At day 42, all hamsters were challenged intranasally with 10^4.0 TCID50 SARS-CoV-2, strain BetaCoV/Munich/BavPat1/2020. At day 46, half of the animals per group (4 out of 8) were sacrificed and at day 49 the remaining 4 animals were sacrificed. (A) Experimental setup and timeline of the hamster challenge experiment. (B) Total IgG anti-spike antibody levels were measured in sera (dilution 1:4,000) from day 42 in an ELISA. (C) Virus neutralization was determined in sera from day 42. (D) When animals were sacrificed at day 46 (day 4 post challenge) and day 49 (day 7 post challenge), the percentage of the lung that presented lung lesions was quantified. The viral load was determined in throat swabs (E), lungs, and nasal turbinates (F). Data are depicted as mean ± SD. Significance (E) is depicted as *p < 0.05, **p < 0.01, ***p < 0.001. Statistical significance of the difference was evaluated by a 2-way ANOVA test followed by the non-parametric Mann whitney test.