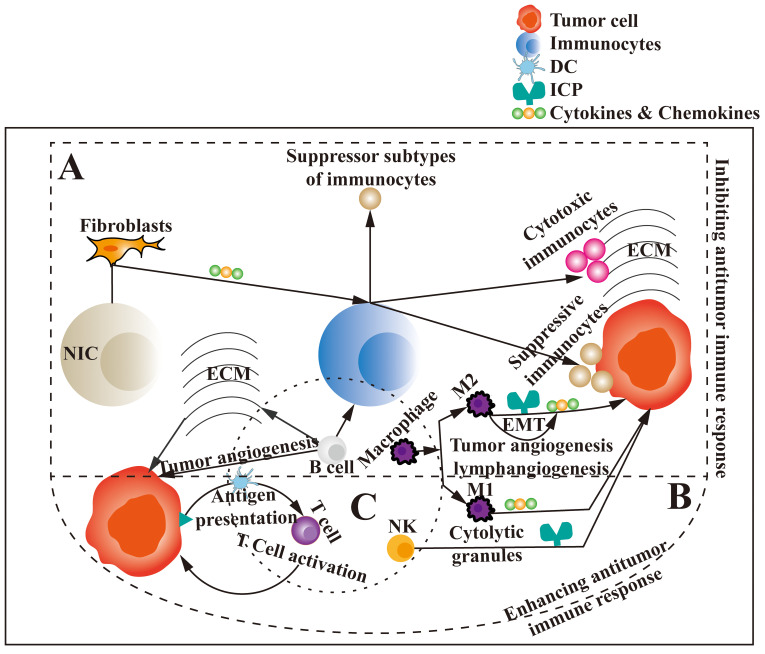
Figure 1.
Associations and function of TIME components. TIME, an extremely complex microenvironment, which is mainly composed of tumor cells, immunocytes, NICs, stroma, cytokines, chemokines, ICPs and the interactions among them, plays a key role in the occurrence, development and metastasis of tumors. NICs, such as fibroblasts, induce the differentiation of immune cells to inhibitory subtypes by producing cytokines and chemokines, which attract the inhibitory immune cells to the tumor center and the cytotoxic immune cells, such as macrophages, T cells and NK cells, to the tumor matrix. B cells promote the growth and metastasis of tumors by inhibiting the function of immune cells, promoting the production of ECM and tumor angiogenesis. Macrophages promote tumorigenesis, progression and metastasis by differentiating into the M2 subtype to express ICPs, secrete cytokines and chemokines, and promote EMT, tumor angiogenesis and lymphangiogenesis. Therefore, the TIME can inhibit antitumor immune responses (part A). However, M1-type macrophages exert antitumor activity by secreting cytokines and chemokines. NK cells can induce tumor cell lysis by expressing immune checkpoints, releasing cytolytic granule perforin and granular enzyme. DCs present tumor antigens to T cells, which can induce T-cell activation to kill tumor cells. In conclusion, the TIME can enhance antitumor immune response (part B). Immune cells, composed of T cells, B cells, NK cells, macrophages and DCs (part C), can each be divided into several different or opposite subtypes that all play a role in inhibiting antitumor immune response or enhancing the different functions of antitumor immune response to inhibit or promote the occurrence, development and metastasis of tumors. TIME, tumor immune microenvironment; NICs, non-immune cells; ECM, extracellular matrix; ICP, immune checkpoint; NK, natural killer; DCs, dendritic cells; EMT, epithelial-to-mesenchymal transition.