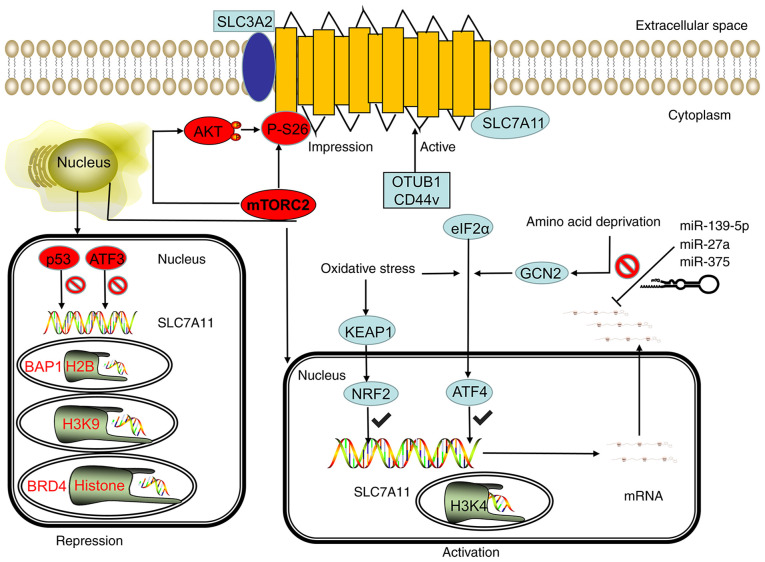
Figure 2.
SLC7A11 is regulated by transcriptional, post-transcriptional and post-translational mechanisms. Under stress conditions, such as oxidative stress and amino acid deficiency, the GCN2-eIF2α and KEAP1-NRF2 signal axes can induce SLC7A11 transcription, and the SWI/SNF chromatin remodeling complex can further promote the NRF2-mediated transcriptional activation of SLC7A11. However, p53 and ATF3 inhibit the expression of SLC7A11 under basal conditions. In epigenetics, the methylation of H3K4 can activate the transcription of SLC7A11, whereas the methylation of H3K9me3 and H3K27me3 can inhibit the transcription activity of SLC7A11. BRD4 can inhibit the transcription of SLC7A11 by regulating histone acetylation. The stability of SLC7A11 mRNA can be regulated by microRNA. miR-139-5p, mir-27a, and mir-375 can destroy the stability of SLC7A11 mRNA and inhibit its transcription. mTORC2 can regulate the phosphorylation of SLC7A11 at serine site 26 and inhibit the transport activity of SLC7A11 directly or through the AKT signaling pathway. CD44v and OTUB1 can inhibit the degradation of SLC7A11, thereby stabilizing the SLC7A11 protein and improving its transport activity. GCN2, general control nonderepressible 2; eIF2α, eukaryotic initiation factor 2α; KEAP1, kelch-like ECH-associated protein 1; NRF2, nuclear erythroid 2-related factor 2; mTORC2, mammalian target of rapamycin complex 2; OTUB1, OTU deubiquitinase ubiquitin aldehyde binding 1; ATF, activating transcription factor; p53, tumor protein 53; SWI/SNF, switch/sucrose non-fermentable modeling complex; BRD4, bromodomain-containing protein 4; miR, microRNA; CD44v, CD44 variant; SLC7A11, solute carrier family 7 member 11; SLC3A2, solute carrier family 3 member 2.