Table 1.
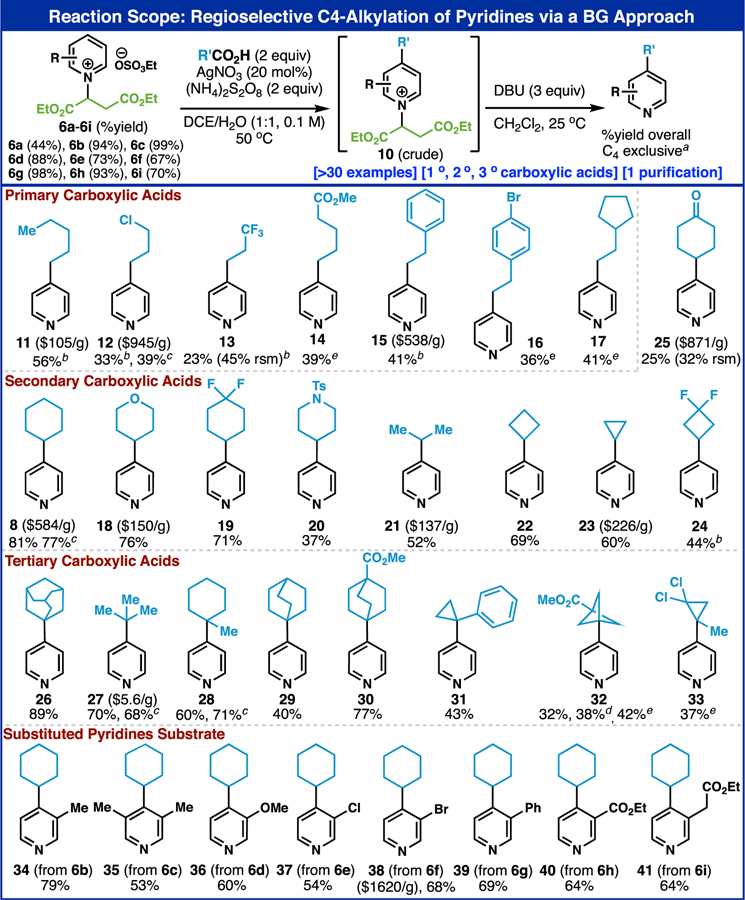
Reaction scope: regioselective C–4 alkylation of pyridines via a BG approach. Yields of 6b–6l are based on isolated yield from substituted pyiridines (two steps). a) 6a (0.5 mmol), carboxylic acid (1.0 mmol), AgNO3 (20 mol%), (NH4)2S2O8 (1.0 mmol), DCE: H2O=1:1, 0.1 M, 50 °C, 2 h. The regioselectivity was determined by crude NMR after first step and confirmed again after final purification step. b) using carboxylic acid (2.0 mmol, 4 equiv) on the Minisci reaction step and DBU (3.0 mmol, 6 equiv) on the removal step. c) 5.0 mmol scale reaction. d) carboxylic acid was used as a limiting reagent. e) performed in 0.3 M. See Supporting Information for detailed experimental procedures.
|
