Figure 1.
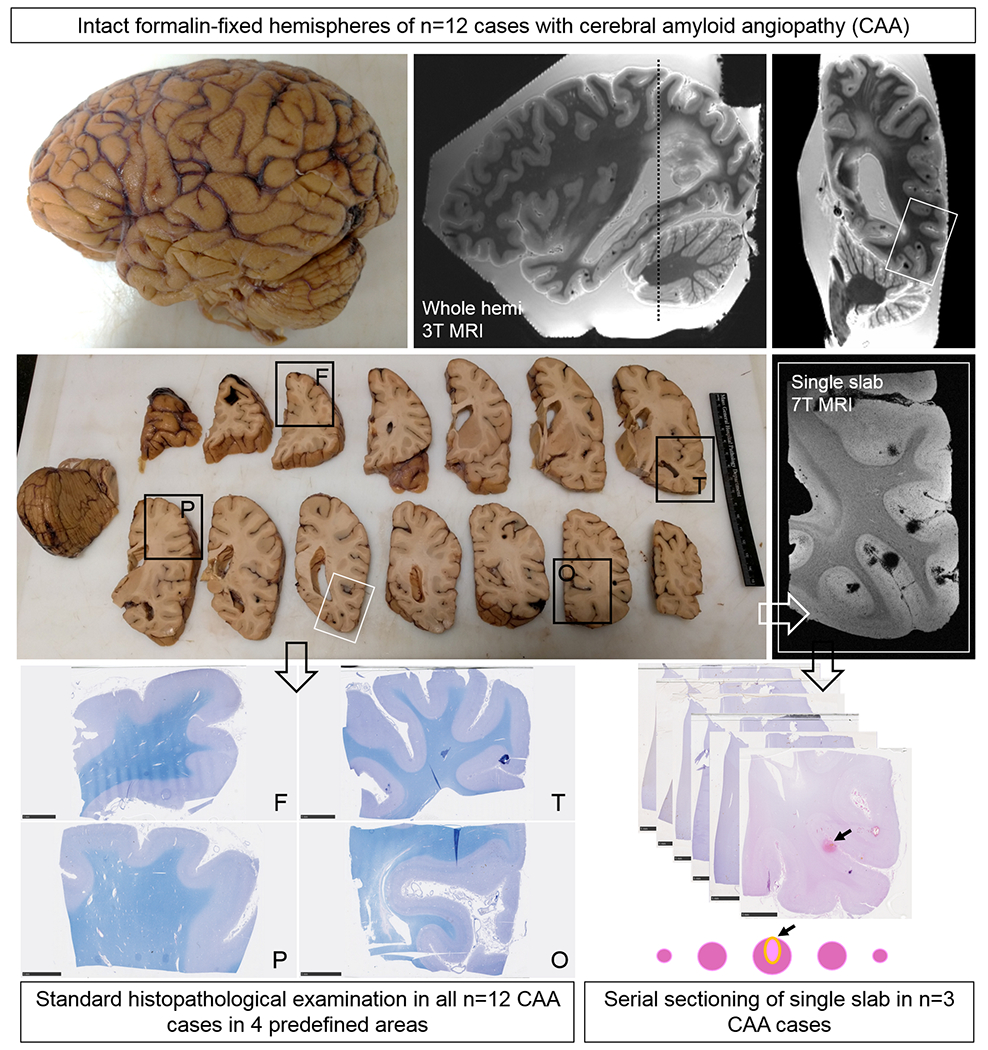
Study design.
Intact formalin-fixed hemispheres of 12 CAA cases were subjected to high-resolution ex vivo 3T MRI to detect microbleeds and microinfarcts. Next, the hemispheres were cut in 10 mm-thick coronal slabs and samples were taken from pre-defined standard areas of frontal (F), temporal (T), parietal (P), and occipital (O) cortex, to fit a standard tissue cassette (black squares). Four adjacent 6 μm-thick sections were cut from these samples and stained with hematoxylin & eosin (H&E) or Luxol fast blue H&E (depicted here) and underwent immunohistochemistry to detect Aβ and GFAP. Finally, three additional samples were taken from three cases in an area with multiple microbleeds (n=2, from temporal cortex) or microinfarcts (n=1, from parieto-occipital cortex) guided by the ex vivo 3T MR images (white square). These samples underwent ultra-high-resolution ex vivo 7T MRI scanning to confirm the high number of lesions in those areas. After 7T MRI, the samples were cut in half to fit two standard tissue cassettes and underwent complete serial sectioning. Sections 1, 21, 41, 61 etc. were stained with H&E to identify microbleeds and microinfarcts, and sections 2, 6, 10, 14 etc. underwent immunohistochemistry against Aβ. If present, the vessel that could be traced through the core of the lesion was identified as the presumed culprit vessel. In addition, for each identified lesion on H&E, sections adjacent to a microbleed or microinfarct underwent immunohistochemistry to detect fibrin(ogen) or SMCs.
