Figure 5.
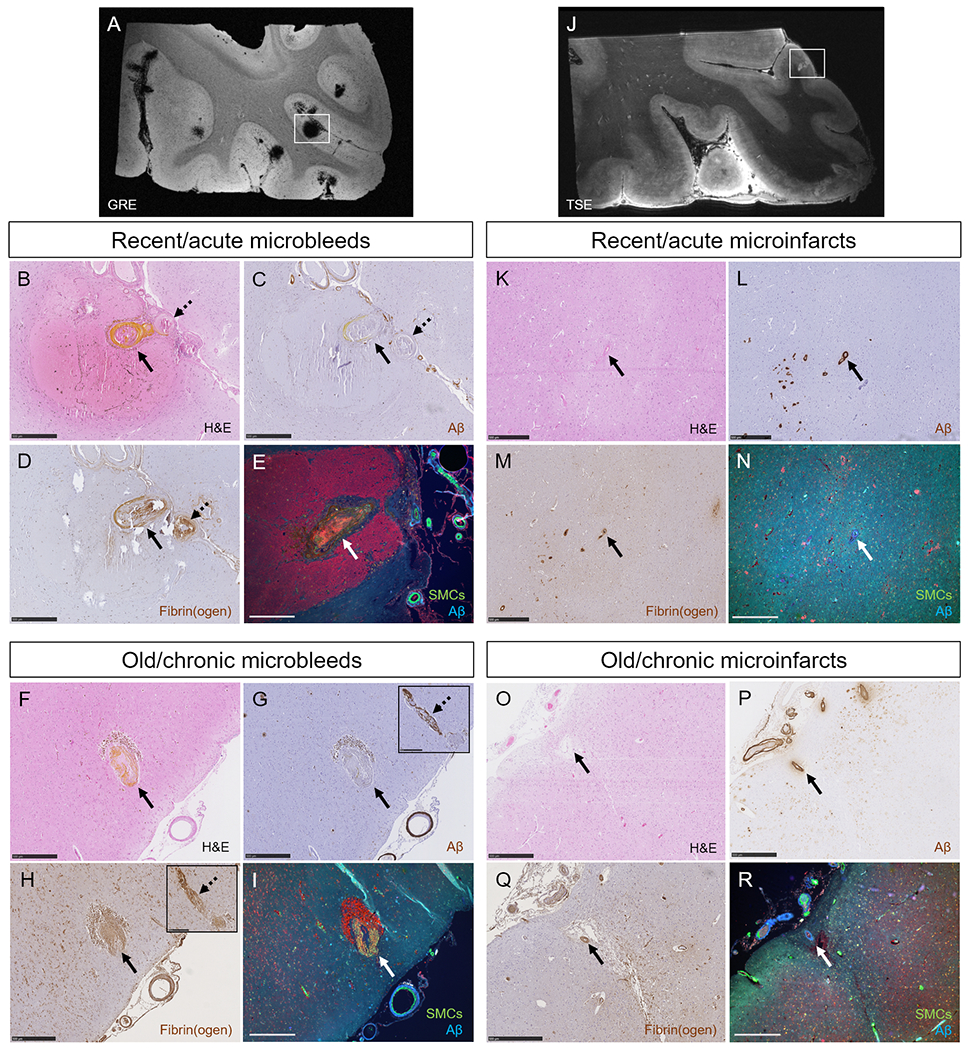
Single-vessel pathologies of microbleeds and microinfarcts.
The high numbers of microbleeds in the additional samples taken from the temporal cortex in two CAA cases were confirmed with ex vivo 7T MRI (A). Single-vessel analysis on serial sections revealed for recent/acute microbleeds: absence of Aβ from the vessel wall at the site of bleeding (BC, arrow, yellow substance is hematoidin). In this example also no Aβ was observed upstream from the bleeding site (C, broken arrow). Extensive fibrin(ogen) was observed at the rupture site and upstream (D). No intact SMC was observed in the responsible vessel (E). Similar observations were made for old/chronic microbleeds (F, brown deposits are hemosiderin-containing macrophages). Note that for this example, Aβ was not present at the rupture site (G, arrow), but was observed downstream (G, broken arrow, inset shows the same vessel captured on a consecutive serial section). Extensive fibrin(ogen) was observed both at the rupture site (H, arrow) and downstream (H, broken arrow, inset shows the same vessel captured on a consecutive serial section), but no SMCs (I). The high number of microinfarcts in the additional sample taken from the parieto-occipital cortex in one CAA case was confirmed with ex vivo 7T MRI (J). Single-vessel analysis on serial sections revealed for recent/acute microinfarcts (K): presence of Aβ in the wall(s) of vessel(s) at the core of the lesion (L), mild fibrin(ogen) deposition (M), and loss of SMCs (N). Similar observations were made for old/chronic microinfarcts (O-R). All scale bars are 500 μm. Note that the lesion in panel E is a different recent/acute microbleed than in panel B, C, and D.
