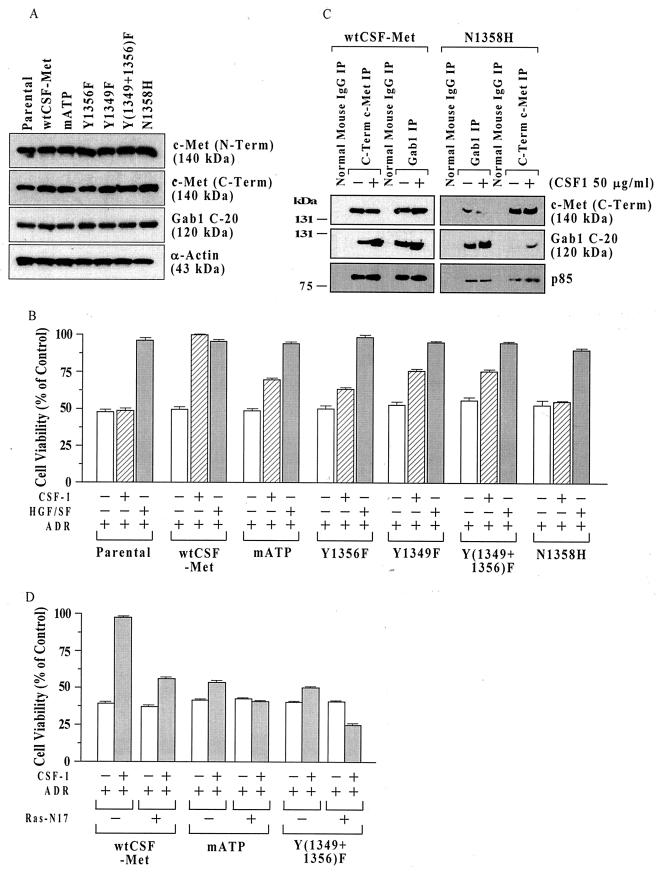
FIG. 1.
Structural requirements for c-Met signaling for cell survival in MDCK cells. (A) c-Met protein levels in cell lines expressing different chimeric Met receptors. Subconfluent proliferating cells were harvested, and equal aliquots of total cell protein (50 μg per lane) were Western blotted, using antibodies directed against c-Met (N-Term), c-Met (C-Term), the multisubstrate adapter Gab1, and α-actin (control for loading and transfer). (B) MTT assays. Parental MDCK cells or clones expressing wt or mutant chimeric CSF-Met receptors were tested for protection against ADR by the chimeric receptor. Subconfluent proliferating cells in 96-well dishes were sham treated (negative control) or preincubated with recombinant human HGF/SF (100 ng/ml; positive control) or recombinant human CSF-1 (50 ng/ml) for 48 h, exposed to ADR (15 μM for 2 h), washed, postincubated in fresh drug-free culture medium for 72 h, and assayed for cell viability using the MTT assay. Cell viability values (relative to sham-treated controls) are means ± standard errors of 10 replicate wells. The survival of untransfected parental cells was not altered by CSF-1, while in wt CSF-Met cells, the protection by CSF-1 was similar to that of HGF/SF. For cells with mutant CSF-Met but not for wt CSF-Met cells, the viability of CSF-1–ADR-treated cells was less than that for HGF/SF-ADR-treated cells (P < 0.001, two-tailed t tests). (C) Decreased association of Gab1 and p85 with N1358H mutant Met receptor. Subconfluent proliferating cultures of MDCK cells expressing wt CSF-Met or N1358H mutant Met receptors were treated with or without CSF-1 (50 ng/ml for 48 h) and subjected to IP using antibodies against c-Met (C-Term), Gab1, or normal mouse IgG as a control. IPs were Western blotted for c-Met (C-Term), Gab1 (C-20), or p85 (the catalytic subunit of PI3 kinase). (D) Inhibition of protection by mATP and Y(1349+1356)F receptors by DN Ras. Subconfluent proliferating cells in 100-mm-diameter dishes were transfected overnight without or with 10 μg of a DN Ras expression vector (Ras-N17), washed, subcultured into 96-well dishes, preincubated with or without CSF-1 (50 ng/ml for 48 h), exposed to ADR (15 μM for 2 h), postincubated for 72 h, and assayed for MTT dye conversion. Ras-N17 caused a significant reduction in the survival of CSF-1-treated cells for all three cell types (P < 0.001) but did not affect the cell survival in the absence of CSF-1.