Abstract
A novel coronavirus disease (COVID-19), caused by a severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), was discovered in Wuhan, China, in December 2019, and the world has suffered from a pandemic. As of 22nd March 2020, at least 185 countries worldwide had been affected by COVID-19. SARS-CoV-2, leading to COVID-19 pneumonia, infects cells through ACE-2 receptors. The disease has different clinical signs and symptoms, including chills, high fever, dyspnea, and cough. Other symptoms including haemoptysis, myalgia, diarrhoea, expectoration, and fatigue may also occur. The rapid rise in confirmation cases is severe in preventing and controlling COVID-19. In this review, the article will explore and evaluate the insights into how COVID influences patients with other comorbid conditions such as cardiovascular disease, diabetes, Parkinson’s, and how conditions Urolithiasis, anosmia, and anuria may develop after infection. The virus mutates and the variants are now prevalent in the present scenario where the world stands in eradicating the pandemic by looking into the development of vaccines by several countries and how the vaccination can temporarily help prevent COVID spread.
Keywords: Coronavirus disease 2019 (COVID-19), World Health Organization (WHO), Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), Cardiovascular disease (CVD), Renin–angiotensin–aldosterone system (RAAS), Hypertension (HTN), Angiotensin-converting enzyme 2 (ACE2)
Introduction
COVID-19 is a global pandemic, highly transmittable infectious disease caused by a strain of novel coronavirus (Thapa et al. 2021). It belongs to a subfamily Orthocoronavirinae, Coronaviridae family, and Nidovirales order, and overall, it has affected the lives of billions of people (Pal et al. 2020). The coronavirus family includes four subgroups (alpha, beta, gamma, and delta) (Ahmad and Rodriguez-Morales 2019). This epidemic began in Wuhan city, China, and the number of cases outside China exceeded the number in China by 15 March 2020 and elevated at an exponential rate (Clerkin et al. 2020). On 7th January 2020, the World Health Organization (WHO) named it a severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) (Lai et al. 2020). WHO declared COVID-19 as a pandemic on 11th March 2020 (Tison et al. 2020).
Human Coronavirus (HCoV) have seven types from which 229E, NL63, OCY3, and HKU1 are associated with upper respiratory tract infection, and SARS (severe acute respiratory syndrome) CoV, MERS (Middle East Respiratory Syndrome) CoV, and SARS-CoV-2 are associated with infection of the lower respiratory tract and acute respiratory distress syndrome (ARDS). Highly pathogenic HCoV has been observed in the last 2 decades, including SARS-CoV in 2002 and 2003 in Guangdong (China), where 8000 cases were reported worldwide and 10% death rate was observed, and MERS-CoV in Saudi Arabia in 2012, which resulted in 2500 positive cases and a 36% mortality rate (Kahn and McIntosh 2005; Srivastava 2020). SARS-CoV and CoV-2 affect humans by entering the host receptor (cellular and angiotensin-converting enzyme 2 receptor) by spike glycoprotein binding (Ni et al. 2020). SARS-CoV has come from bats, similar to other coronaviruses, as it shares a nuclear identity of 89–96% with bat coronaviruses. Like SARS and MERS, the SARs-CoV-2 is also reported to be a Malayan Pangolin whose nuclear identity shares 91% that of humans, possibly from bats to intermediate host (Clerkin et al. 2020). Cases were reported to WHO on 31st December 2019, and on 30th January 2020, the COVID-19 was declared a global health emergency (Walls et al. 2020). The virus constitutes a particular risk to people above 60 years of age and those with underlying medical conditions, such as diabetes, cancer, chronic respiratory disease, and cardiovascular disease (CVD). It has an incubation period of 2 days to 2 weeks, showing symptoms such as fever, chills, shortness of breath, sore throat, loss of taste and smell, fatigue, cough, and pneumonia (considered to be a severe manifestation of COVID-19 (Santesmasses et al. 2020). Rhinorrhea and diarrhea have also been reported in some cases with COVID-19. Most patients (81%) have moderate COVID-19 symptoms, such as mild or no pneumonia.
Moreover, those with significant symptoms were severe symptoms such as dyspnea, blood oxygen saturation < 93%, respiratory rate < 30/min, the partial pressure of arterial oxygen fraction to inspired oxygen proportions < 300, and lung infiltrate > 50% within 24–49 h, and septic shock, respiratory failure, and even organ multiple dysfunctions were critical for 5% (Srivastava 2020). The initial assessments were detected that the SARS-CoV pathogen is present in the upper and lower respiratory tracts. Both symptomatic and asymptomatic individuals can cause transmission, with the secondary range of infections from 0.5 to 5%. The virus is stable, with a median incubation period of 4–5 days, and almost 97.5% of infected persons will experience symptoms within 11.5 days of exposure (Clerkin et al. 2020). This has recommended the guideline of a maximum of 14 days to quarantine potentially exposed individuals to restrict onward spread. At present, the ratio of asymptomatic to symptomatic infections is unknown (Donnelly et al. 2003). The molecular diagnostic test for the SARS-CoV-2 infection remains the real-time reverse transcription-PCR (RT-PCR) assay. At the same time, radiographic findings, such as antibody-based techniques and chest computed tomography (CT), are available (Srivastava 2020). COVID-19 pandemic exponent doing new studies on other medical methods such as CT (chest) and RT-PCR—a costly examination with little access to diagnostic facilities. But the use of RT-PCR tests has a few limitations, such as long amputation times, complicated processing, the necessity of quality-controlled laboratories, costly, and the demands for trained experts (Srivastava 2020; Al-Sadeq and Nasrallah 2020). SARS-CoV-2 has a more robust transmission capability than the SARS-CoV, leading to an SARS outbreak in 2003. However, infections share many symptoms of SARS-CoV, MERS-CoV, and SARS-CoV-2, including myalgia, cough, fever, and dyspnea (Petersen et al. 2020). SARS- and MERS-infected patients have about one-third more involvement of gastrointestinal than SARS-CoV-2 patients. The swift increase in the confirmed cases is severe in preventing COVID-19, with an unparalleled impact on public health and social and economic activities (Di Gennaro et al. 2020). The exponential increase in the number of COVID-19 patients in numerous countries worldwide has affected medical systems in recent months.
Early clinical evidence suggests that COVID-19 susceptibility and results are significantly related to CVDs. The binding affinity of the SARS-CoV-2 with angiotensin-converting enzyme 2 (ACE2) appears to be higher than SARS-CoV, which explains the significantly higher global power of COVID-19 than the initially SARS-CoV. ACE2 is the SARS-CoV-2 receptor cellularly and prime regulator of renin–angiotensin–aldosterone system (RAAS), which has attracted wide attention as the cause of the pandemic. Increased and reduced ACE2 can induce both systemic and pulmonary hypertension, myocardial infarction (MI), heart failure (HF), and diabetic complications (Tadic et al. 2020). However, the uncontrolled infection may cause a cytokine storm in response to respiratory symptoms.
In contrast, proinflammatory cytokines and chemokines such as tumor necrosis factor-α (TNF-α), and interleukin (IL)-1β and IL-6 are overproduced by the immune system causing multiorgan damage (Vishwakarma et al. 2021). In many individuals, COVID-19 causes coagulation disturbances that lead to thromboembolic events (Nishiga et al. 2020). The development of vaccines and therapeutic methods would lead to a better understanding of the biological features of the virus, such as preventing the binding of ACE2 and SARS-CoV-2 virus. The main benefit here is that there is no change in host ACE2 protein; therefore, there is no fear of beneficial changes that could hinder drug or vaccine development (Vellingiri et al. 2020).
Structural assembly of novel strain: the SARS-CoV-2
SARS-CoV-2 is a zoonotic virus belonging to the β-coronavirus genus consisting of crown-shaped peplomers, enveloped as positive-single-strand RNA (+ ssRNA) viruses, identified in the pleomorphic form with a size of 80–160 nm and a genome varying between 27 and 32 kilobases (kb) (Vellingiri et al. 2020; Ji et al. 2020). The virus has four major structural proteins to control the viral structure and function: (1) the protein Envelope (E), (2) the protein Nucleocapsid (N), (3) the protein of the Membrane (M), and (4) the protein Spike (S). The significant characteristic of the coronavirus is because of S-proteins as it forms a crown-like structure on the outermost layer. There are three main parts of the S-protein, a large ectodomain, a short intracellular tail, and a single-pass transmembrane anchor, and it plays an essential role in the host cells’ viral entry. There are two subunits of the ectodomain, the receptor-binding subunit (S1) and the membrane fusion subunit (S2), which also has three domains (A, B, and C). The host receptor binding is the responsibility of subunit S1 domain A present on CoV-HKU1 and CoV-OC43, while both domains A and B are used by MERS-CoV to infect humans. The structure of the S-protein is almost identical in both SARS-CoV-2 and SARS-CoV variants. However, via direct interaction with domain B and also having more than 70% same genetic sequence, both SARS-CoV, and SARS-CoV-2 are using alike receptor (ACE2) for cellular entry (Ji et al. 2020; Samudrala et al. 2020). This in turn attached directly to the ACE2 in the host cells.
Pathogenesis
ACE2, an aminopeptidase that is membrane-bound and plays a vital role in the immune system and CV system. ACE2 lining the microvasculature is majorly expressed in pericytes, followed by fibroblasts, cardiomyocytes, and vascular smooth muscle cells (Zheng et al. 2020a, b). The virus’s spike protein binds to the host ACE2 receptors, initiating SARS-CoV-2 infection, following the activation of transmembrane serine protease 2 (TMPRSS2). A further entry in macrophages, lungs, particularly in type II alveolar cells, and other cell types, SARS-CoV-2 binds to an ACE2 transmembrane protein (Clerkin et al. 2020). This process demands that the viral S-protein is primed by the cathepsins (cathepsin B and cathepsin L) and TMPRSS2 encoded by the genes CTSL and TMPRSS2, respectively, TMPRSS2 was found to be moderately expressed throughout all cardiac types of cells, whereas CTSL expression was decreased in adipocytes, cardiomyocytes, fibroblasts, and macrophages (Unudurthi et al. 2020). As a result, SARS-CoV-2 infection necessitates the ACE2 and TMPRSS2 expression in the identical type of cell, as proteolytic cleavage of the viral protein S is required for the ACE2 virus bindings.
The entry of the SARS-CoV into the host cells, the virus attaches ACE2 throughout the membrane invagination and fusion causes the downregulation of ACE2 (Tomasoni et al. 2020). A SARS mouse model shows that ACE2 levels are significantly reduced in the heart after the SARS-CoV infection. In addition, ACE2 shows beneficial effects on the lungs. In the normal adult lungs, ACE2 is manifested in the alveolar type II epithelial cells, and to decrease surface tension and prevent the alveoli from collapsing, these cells produce surfactant proteins. ACE2 knockout aggravated acute lung injury shown in a preclinical study, but treatment with recombinant ACE2 reversed the harm (Nishiga et al. 2020). Therefore, SARS-CoV-2 results in ACE2 downregulation similar to the SARS-CoV, which leads to lung damage exacerbation and atherosclerosis progression (Nishiga et al. 2020; Tomasoni et al. 2020). In states with excessive RAAS activation, such as atherosclerosis, HTN, and congestive heart failure (CHF), ACE2 is expressed primarily in the heart and works to counteract the effect of angiotensin II. In addition, to the lungs and heart, ACE2 protein is predominantly present in the intestine, kidney, and vascular endothelium, the coronavirus’s primary targets (Vellingiri et al. 2020). There is rising evidence that COVID-19 infection is associated with increased CVD mortality and morbidity.
COVID-19 and cardiovascular diseases
Heart diseases were prevalent comorbidity in COVID-19 cases with SARS and MERS predecessors. CVD and diabetes mellitus (DM) were found to be 8% and 11% in SARS infection, and the existence of either comorbidity enhanced the risk of death by a factor of 12. In about half of the MERS cases, DM and hypertension (HTN) were present, and approximately 30% of the cases included CVD (Samudrala et al. 2020). The increased prevalence of cardiovascular comorbidities also relates to COVID-19, particularly those with more severe diseases (Clerkin et al. 2020).
According to recent studies, older individuals with chronic illnesses, such as CVD, have a higher risk for COVID-19-related morbidity and mortality than the general population. However, respiratory symptoms are the most prevalent clinical manifestations of COVID-19, CV involvement can arise through various pathways (Paramasivam et al. 2020). In cases with pre-existing cardiovascular disease, COVID-19 can cause heart attacks or lead to CHF. This occurs due to a severe viral illness’s combination and needs higher on the heart, an elevated heart rate aggravated by reduced oxygen levels due to myocarditis, respiratory symptoms, and a higher risk of blood clot formation. Further, an increase in these heart problems, COVID-19 patients have also developed a more unusual condition known as myocarditis. COVID-19 infection also presents a potential risk to heart transplantation cases, influencing donor selection, immunosuppression, and post-transplant therapy. The mechanism underlying such associations is still unknown. VD is more frequent in cases with advanced age, higher ACE2 levels, functionally compromised immune systems, and patients with CVD who are predisposed to COVID-19 infection (Clerkin et al. 2020). Coagulatory anomalies were also found in COVID-19 individuals, which led to blood clots and, eventually, multiorgan dysfunction, including HF. The activation of a cascade of proteins, including thrombin, coagulation factors, and fibrinogen, causes coagulation. When blood clots are formed, uncommon enzymes such as plasmin are regularly degraded, leading to the formation of fibrinogen-degradation products (FDPs) (Unudurthi et al. 2020). Pulmonary embolism (PE) and disseminated intravascular coagulation (DIC), both characterized by higher D-dimer levels and FDPs, are prevalent in the infection with COVID-19. DIC was discovered in 71.4% of non-survivors (Unudurthi et al. 2020).
Independent of COVID-19 infections, lymphocytopenia has been linked to CVD. In a study of 392 subjects with Heart Failure Reduced Ejection Fraction (HFrEF), lymphocytopenia was significantly associated with more severe New York Heart Association (NYHA) Class III and IV cardiac disease compared to milder NYHA Class I and II cardiac diseases. The relative concentration of lymphocytes is now proposed as a diagnostic biomarker in acute or chronic coronary artery disease cases. Lymphocytopenia and CVD do not have a well-established causative relationship. T-lymphocytes secret Interferon-γ during vascular repairing, on the other hand, appears to modify smooth muscle proliferation.
Furthermore, studies in animals lacking T-lymphocytes show more arterial lesions after a vascular injury than the control group (Unudurthi et al. 2020). The Novel Coronavirus Pneumonia Emergency Response Epidemiology Team examined all COVID-19 confirmed cases to China’s Infectious Disease Information System till February 2020. The researchers discovered that the case fatality rate (CFR) was about 0.9% for cases without any comorbid conditions, while there was a higher CFR for cases with comorbid conditions. This included a mortality rate of 10.5%, 7.3%, 6.3%, 6%, and 6% for CVD, DM, chronic respiratory disease, HTN, and cancer patients, respectively (Unudurthi et al. 2020).
COVID-19 and hypertension
The American Heart Association (AHA) and the American College of Cardiology (ACC) define hypertension as systolic blood pressure (BP) > 130 mm Hg and diastolic BP > 80 mm Hg as a significant modifiable risk factor for atherosclerotic CVD (Sharma and Singh 2020a, b, c). The HTN prevalence in COVID-19 is not surprising, also it does not necessarily suggest a causal relationship or severity between HTN and COVID-19, as it is especially prevalent among the elderly, who are at higher risk of SARS-CoV-2 infection (Kanwal et al. 2020). If HTN is a potential risk for SARS-CoV-2 infections, the data show 15 to 40% prevalence estimates, mainly in the high BP population (approximately 30%). Initial COVID-19 hotspot analyses found high rates of HTN among hospitalized COVID-19 patients. An overall HTN rate of 56% was found in a US study of 5700 hospitalized patients, similar to HTN rates of 50% and 49% in China and Italy, respectively. According to a survey, 138 COVID-19 cases were hospitalized in Wuhan, with 31% having HTN. HTN was present in 58% of those seeking ICU admission, compared to 22% who did not have HTN (Wang et al. 2020; Cook 2020). In addition, another study from Wuhan reported that from 191 hospitalized patients, outcomes suggest that 30% had HTN and 48% died having HTN compared with 23% of survivors (Cook 2020; Fang et al. 2020). The human pathogenic SARS-CoV and SARS-CoV-2 coronaviruses bind to their cellular targets through the ACE2, and ACE2 expression is increased significantly in type 1DM and type 2DM patients treated with angiotensin II receptor blockers (ARBs) and angiotensin-converting enzyme inhibitors (ACEIs). HTN, also treated with ARBs and ACEIs, causes an ACE2 upregulation. ACE2 is required for RAAS and is also implicated in the pathophysiology of Hypertension. Numerous experimental studies have shown RAAS inhibition with ARBs and ACEIs to result in a compensatory rise in ACE2 at tissue levels, indicating that these medications may be harmful to SARS-CoV-2. It is also important to note that no conclusive evidence or research has proved that ARBs and ACEIs raise ACE2 levels in body tissue (Paramasivam et al. 2020; Banerjee et al. 2021). Several learned organizations, such as the European Society of Cardiology, the International Society of Hypertension, and the European Society of Hypertension already have officially endorsed this, implying that there is no need to resist ARBs or ACEIs in patients who are at risk of COVID-19 infection. Losartan inhibits the effect of angiotensin II on ACE2 internalization and intracellular degradation, suggesting that ARBs can protect cells from viral infection. Ibuprofen and thiazolidinediones can also raise ACE2. These findings indicate that ACE2 expression is elevated in DM and that therapy with ARBs and ACEIs increases ACE2 expression. COVID-19 infection would be aided by increased ACE2 expression. Therefore, there can be a hypothesis that treatment with ACE2-stimulating drugs for diabetes and hypertension increases the risk of severe and fatal COVID-19 growth. The risk of severe COVID-19 infection is higher for patients with heart disease, hypertension, or diabetes treated with ACE2-increasing medicinal products and should be checked for ACE2-modulating medicinal products, such as ACEIs or ARBs (Fang et al. 2020; South et al. 2020).
In HTN and COVID-19, the dysregulated immune system seems to be another mechanism connecting both HTN and COVID-19. More immune system dysregulation can result from inadequate blood pressure control. It has been demonstrated that it is linked to the number of circulating lymphocytes in humans. In patients with hypertension, there is evidence of CD8+ T cell dysfunction. These immunosenescent CD8+ T cells are insufficient to effectively battle viral pathogens resulting in cytokine overproduction, a scenario that could lead to a link to COVID-19. It is also possible that ARBs or ACEIs can help to restore the immune system’s dysregulation in HTN, at least partly, by improving BP control (Guzik et al. 2020). Although HTN is overrepresented in severely ill and hospitalized patients with COVID-19, it is unclear whether this association is causal or confounded by aging and other HTN-related comorbidities such as chronic kidney disease (CKD), obesity, and diabetes mellitus (DM) (Guzik et al. 2020).
COVID-19 and myocardial injury, myocarditis
Cardiac dysfunction is a rare complication of COVID-19 infection. Moreover, many infected patients have developed myocardial disease, and this is not the only time a coronavirus has been linked to CV problems. SARS viruses have resulted in diastolic and systolic dysfunction, HF, arrhythmias, and sudden cardiac collapse due to MI (Babapoor-Farrokhran et al. 2020). The ‘cytokine storm,’ systemic hyper-inflammation induced in the lungs by SARS-CoV-2, can cause heart damage in the long run. Several clinical studies of COVID-19 have found markedly increased inflammatory biomarkers in the blood, including TNF-α, IL-2, IL-7, interferon-γ inducible protein (IP)-10, IL-6, monocyte chemoattractant protein-1 (MCP-1), procalcitonin, C-reactive protein (CRP), ferritin, and macrophage inflammatory protein 1-α (MIP-1α). Higher inflammatory cytokines systematically, caused by a local infection in the lungs, lead to maladaptive remodeling and inflammatory pathways in various organs, such as the heart. The development of cardiovascular disease is linked to increases in proinflammatory cytokines. The anomalous expression of proinflammatory cytokines such as IL-1, TNF-α, IL-6, and matrix metalloproteinase (MMP) in the heart, for instance, is believed to contribute to the MI onset (Unudurthi et al. 2020).
In most cases, lactate dehydrogenase (LDH) and serum creatine kinase (CK) levels are raised in nearly all hospitalized COVID-19 patients. Furthermore, multiple studies have shown that COVID-19 infection can lead to cardiac complications like fulminant myocarditis. In a study of hospitalized Chinese patients, HF was identified in 23% of COVID-19 patients. Only 12% of survivors had HF, according to almost half of non-survivors. Evidence of myocardial injuries, such as a rise in cardiac troponin I (cTnI) high-sensitivity (> 28 pg/mL), were found in 5 of Wuhan’s initial 41 COVID-19 cases (Guzik et al. 2020). Troponin elevations may occur due to a variety of reasons during a COVID-19 outbreak. Roughly data show 8–12% of confirmed cases develop significant cTnI high levels (Bansal 2020). In a multi-center cohort study of 191 COVID-19 infected patients, 17% (33 patients) suffered from acute HF, with 32 of them dying (Nishiga et al. 2020). Till date, there is a lot of debate about the function of the CoV-2 cellular docking receptor for ACE2, affected by CVD and its therapy. In patients with chronic stable CVD, particularly when HF is challenging, a rise in cTnI is common, showing a worse prognosis. Moderate cTnI elevations during infection may only be a proxy marker for the severity of pre-existing CVD. In patients with COVID-19, myocardial injury can result from plaque break, cytokine storm, hypoxic injury, microthrombi, coronary spasm, or direct endothelial or vascular injury.
Acute myocarditis has a wide range of clinical incidence and is a significant diagnostic challenge in the COVID-19 period. The SARS-CoV-2 infection, unlike other infections like the coxsackie virus, is not reported to be cardiotropic. The virus has an affinity to bind with the ACE2 receptor, which is found in both the lungs and the heart. A cytokine release storm develops when T cell activation is imbalanced, resulting in the inappropriate release of cytokines such as IL-17, IL-6, and others, potentially causing substantial cellular damage (Sharma et al. 2021a, b). Moreover, this strong immune response may cause plaque instability, leading to acute coronary syndrome (ACS) development (Long et al. 2020). There seem to be no reports verifying myocarditis induced by SARS-CoV-2 via polymerase chain reaction (PCR) histological and viral genomic analysis. However, molecular evidence via a biopsy is still needed to detect the SARS-CoV-2 genome within myocytes. In addition, a COVID-19 case with regional wall motion anomalies had a biopsy with consistent lymphocytic myocarditis, but biopsy analysis of histopathological and viral genomic PCR revealed that the SARS-CoV-2 viral genome was not found inside the myocytes (Rehman et al. 2020).
Serum troponin values are irregular in myocarditis and myocardial injury patients. An electrocardiogram (ECG) can show a variety of results, sometimes mimicking ACS. Abnormalities of non-specific ST segment-T wave, alteration of the T wave, and variants (depression and elevation) of the PR and ST segments are among the ECG anomalies caused by myocardial inflammation (Long et al. 2020). Echocardiography and cardiology consultation are encouraged if one is available, as it is difficult to distinguish between myocarditis and ACS. Echocardiographic evaluation is much more likely to detect a focal wall motion irregularity with active, significant ACS, while severe types of myocarditis related to COVID-19 infection indicate either no wall motion abnormalities or global wall movement dysfunction (Rehman et al. 2020). Case reports also indicate that even without the clinical features of respiratory infections, myocarditis can occur with COVID-19. Myocarditis results in myocardial inflammation, necrosis, and ultimately ventricular dysfunction, either focal or global (Rehman et al. 2020).
There is also minimal histological proof of myocardial damage or myocarditis in COVID-19. According to a case report, SARS-CoV-2 may invade the myocardium directly due to low-grade myocardial site and inflammation of coronavirus outside the cardiomyocytes, which is determined by endomyocardial biopsy. As per clinical evidence, progressive systemic inflammation is the primary cause of myocardial injury. A small proportion of patients infected with COVID-19 develop viral myocarditis because the virus can infect the myocardium (Nishiga et al. 2020).
COVID-19 and cardiac arrhythmias
Arrhythmia is a common condition in COVID-19-infected patients, and available evidence suggests that 20–36% of COVID-19-infected patients suffer from acute myocardial injury, with a higher death rate than those who do not have a cardiac injury, proportional to the level of cTnI elevation (Ni et al. 2020). Furthermore, cardiac arrhythmias, including malignant ventricular arrhythmias (VAs), affect 6–17% of patients, increasing prevalence (44%) in ICU patients. Importantly, while clinically healthy individuals may have a reduced arrhythmiatic prevalence, severely ill cases have a much higher risk of cardiac arrhythmias (Manolis et al. 2020). The COVID-19 virus and its harmful impacts of systemic disease and adverse reactions to medications to treat the pandemic may cause arrhythmias involving life-threatening VAs. During a study of 700 people who had COVID-19 infections, 25 of them had atrial fibrillation (AF), 9 had bradyarrhythmias, 9 had heart attacks, and 10 had non-sustained ventricular tachycardias that didn’t last long enough to be dangerous (NSVTs). Severe in-hospital death was linked to cardiac arrests. Arrhythmias can result from metabolic derangements, hypoxia, acidosis, intravascular volume imbalances, neurohormonal effects, and catecholaminergic stress COVID-19 patients (Manolis et al. 2020).
COVID-19 and diabetes
The underlying mechanisms involved in changes in susceptibility are unknown, studies suggest that higher blood sugar levels cause immunosuppression (Deepika et al. 2019; Singh et al. 2020a, b, c). Other conditions, such as obesity and CVD, are often associated with diabetic cases, enhancing the infection risk. Higher blood glucose levels may be associated with more severe disease manifestations (Singh et al. 2021; Tsatsakis et al. 2020). Diabetes is most commonly associated with unfavorable infections with severe cardiovascular illness, kidney disease, and aging (Singh et al. 2020a, b, c). The correlation between diabetes and COVID-19 is complex; it is unclear whether hyperglycemia leads to the new coronavirus’s virulence or alters carbohydrate metabolism. This disease’s various metabolic inequalities caused this disease result in a proinflammatory and pro-oxidative state in the body, reducing resistance to disease with the novel coronavirus (Cristelo et al. 2020).
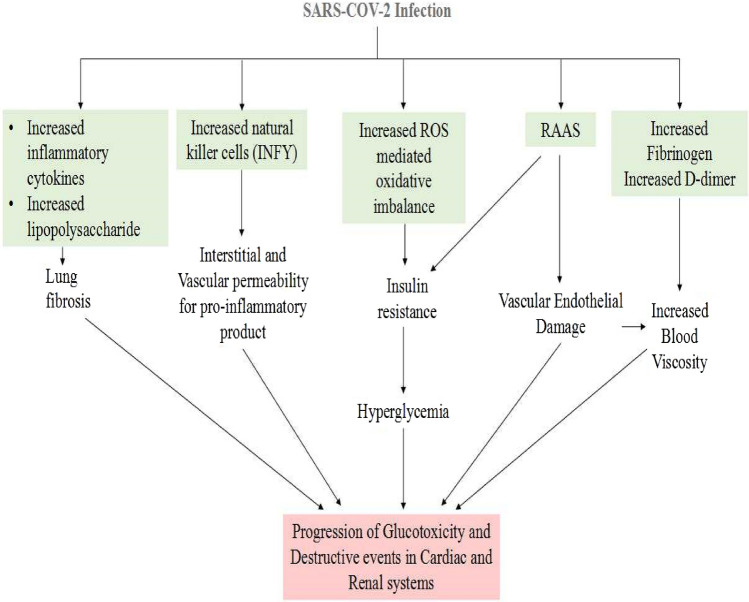
In general, diabetic patients are predisposed to viral infection via impaired neutrophil chemotaxis and phagocytosis; there are other factors involved in increased risk and severity of SARS-CoV-2 infection in diabetes. Increased ACE2 expression is one of the primary factors causally related to COVID-19 infection as ACE2 receptors are also found in pancreatic islets. Another factor is the enhanced levels of type-1 membrane-bound protease, namely furin belonging to the proprotein convertase subtilisin/Kexin family (PCSK). Furin has been involved in the entry of SARS-CoV-2 into the cell, which further potentiates viral replication. Increased functions of T cell and interleukin-6 (IL-6) have been reported in COVID-19 infection with diabetes (Singh et al. 2020a, b, c; Sanyaolu et al. 2020). Figure 1 illustrates COVID-19-associated diabetes mellitus progression and further leads to cardiac dysfunction (Cristelo et al. 2020; Apicella et al. 2020).
Fig. 1.
COVID-19 leads to diabetes mellitus progression and shows cardiac dysfunction
COVID-19 and thrombosis
COVID-19 has been associated with coagulation problems and triggers a high incidence of thromboembolic events and a prothrombotic state that raises concern about unique prothrombotic pathophysiology (Hanff et al. 2020). In early reports of China shows the first evidence of irregular coagulation parameters associated with COVID-19. The baseline characteristics of the first hospitalized 99 patients in Wuhan showed that 6% had increased partial activated thromboplastin time, 5% had increased prothrombin time, and 36% had increased D-dimer time (Lim et al. 2021).
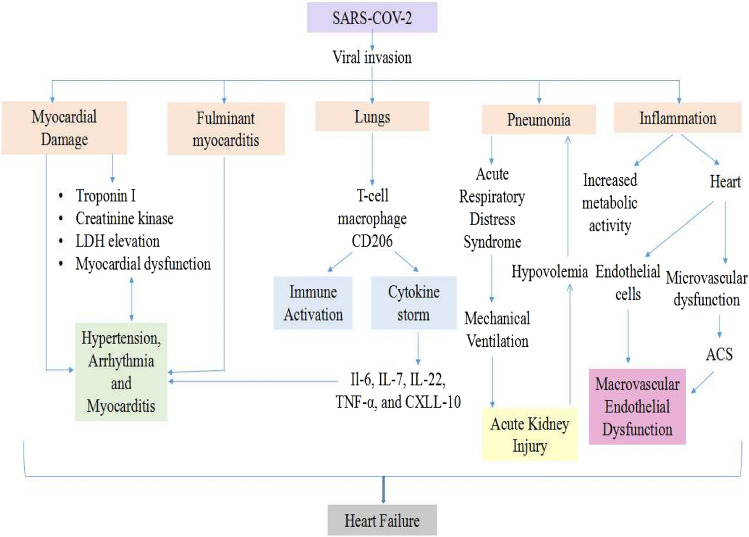
In critically ill patients, venous thromboembolism (VT), which involves PE and deep vein thrombosis (DVT), is a severe complication. However, according to autopsy reports, 7 of the 12 deaths with COVID-19 infection for whom VT was not suspected before death had DVT, while 4 of them had a PE (Nishiga et al. 2020). The activation of subsequent fibrin deposition and coagulation in the early stages of some infections is likely adaptive. Still, chronic inflammation can rapidly result in a significant negative hyperinflammatory response mediated by macrophage activation syndrome and cytokine storm. Cytokine storm is a proinflammatory cytokine release auto-amplifying syndrome that causes ARDS and multiorgan dysfunction in various settings, such as Castleman disease and CAR T cell therapy (Gupta et al. 2020). The prothrombotic condition can also contribute to hypoxia-mediated hyperviscosity and hypoxia-inducible factor 1 (HIF-1) upregulation signaling pathway following acute lung injury. In hospitalized COVID-19 patients, multiple non-specific inflammatory biomarkers are significantly increased, namely erythrocyte sedimentation rate (ESR), ferritin, and C-reactive protein, as are several procoagulants factors such as Factor VIII and Von Willebrand factor. Besides, various proinflammatory cytokines are increased, including IL-2R, IL-6, IL-10, IL-8, and TNF-α (Hanff et al. 2020). The common pathophysiological link between COVID-19 and CVD is shown in Fig. 2 (Clerkin et al. 2020; Tadic et al. 2020; Nishiga et al. 2020; Samudrala et al. 2020).
Fig. 2.
The common pathophysiological link between COVID-19 and CVD
As mentioned above, cardiovascular diseases were/are the prevalent comorbidities in COVID-19; it is of utmost importance to pay attention to the COVID-19-associated risk factors and to spot COVID-19 critically ill patients early to reduce the risk of death in these patients (Zheng et al. 2020a, b; Page and Ariëns 2021). Cardiovascular comorbidities such as hypertension, thrombosis, atrial fibrillation could affect the prognosis of the COVID-19. Biochemical investigations such as WBC, AST, Cr, hs-cTnl, PCT, LDH, and D-DIMER could infer the COVID-19 progression (Rostami and Mansouritorghabeh 2020; Cui et al. 2020; Zhou et al. 2020). Literature data reveal the involvement of ACE2 (angiotensin-converting enzyme) as the receptor for the SARS-CoV-2 entry. ACE-2 is widely expressed in the cardiovascular and lungs and other organizations such as kidneys, colon, testes, endothelium, and pancreas. The primary role of ACE2 is to incise Ang-II to release Ang 1–7, which mediates the protective effects of vasodilation, anti-proliferation, and anti-inflammatory and to antagonize Ang-II induced vascular smooth muscle contraction, fibrosis promotion, cell proliferation, and vascular inflammation. Ang-II is the critical player in regulating the RAAS system.
RAAS (renin–angiotensin–aldosterone system) plays a vital role in the pathogenesis of cardiovascular diseases such as hypertension and myocardial infarction (Zheng et al. 2020a, b; Singh et al. 2020a, b, c). S-glycoprotein on the surface of SARS-CoV-2 binds to ACE-2 receptor causing conformational changes in the S-glycoprotein and attacking the alveolar epithelial cells (Singh et al. 2020a, b, c). Binding leads to the downregulation of ACE2 expression in the lungs via internalization, shedding, and viral replication. Downregulation of ACE2 expressions results in increased concentration of Ang-II. Increased Ang-II promotes the inflammatory response, exudation of macrophages and neutrophils, resulting in difficulty maintaining oxygenation and further resulting in loss of pulmonary function. Conformational changes in the S-glycoprotein allow the proteolytic digestion by host cell enzymes, namely TMPRSS2 and Furin (proteases) (Fernandez et al. 2018; Singh et al. 2020a, b, c). Literature reveals the central role of inflammation in the development of cardiovascular diseases.
In cardiovascular diseases such as hypertension, atherosclerosis, inflammation is already present. Furthermore, inflammatory storm induced by a viral infection in patients with cardiovascular dysfunction leads to an increase in inflammatory factors such as interleukins-4, -6, -8, and -10, TNF-α, CRP, cytokines, and platelet cytokine release, including lipopolysaccharide contributing to increase plasma cytokine levels causing systemic injury which could further worsen the cardiovascular health and may lead to multiple organ failure and even death. Cytokine storm is associated with increased ferritin levels and hemodynamic instability leading vascular damage leading to multiple organ failure. IFN-gamma production and modulated natural killer cell activity increase the vascular permeability for proinflammatory products. ROS production increases in COVID-19 infection. Abnormal activation of the RAAS system and enhanced ROS production cause vascular endothelial damage contributing to cardiovascular events such as thromboembolism, disseminated intravascular coagulation (DIC) (Singh et al. 2020a, b, c; Zheng et al. 2020a, b; Lim et al. 2021). When patients have combined diseases such as hypertension and diabetes, the body is put under a lot of stress for long periods, and their immunity suffers.
Furthermore, a lengthy history of diabetes and hypertension damages the vasculature (Sharma and Singh 2020a, b, c). When combined with a severe viral infection such as COVID-19, it is more likely to get infection into severe disease. Chronic heart disease patients are more likely to be getting an infection due to their low immunity and weakened heart function. Therefore, when they get infected with SARS-CoV-2, they are more prone to suffer from cardiovascular events and develop into critical health. Therefore, underlying diseases such as hypertension, diabetes or cardiovascular diseases are risk factors for disease (COVID-19) progression (Bansal 2020; Nishiga et al. 2020; Zheng et al. 2020a, b).
The presence of abnormal coagulation function is another complication in COVID-19 patients, as discussed above. After viral infection, monocytes and tissue cells get activated, promoting the release of cytokines and expression of tissue factors, causing blood’s hypercoagulability. This could increase the risk of thrombosis and more susceptible to cause ischemia and hypoxia due to embolization of the viscera, leading to critical health progression or even death. D-Dimer levels more than 0.5 mg/L suggest the blood’s hypercoagulability leading to further deterioration of the health of patients infected with SARS-CoV-2. hs-cTNI more than 28 pg/ml in SARS-CoV-2-infected patients suggests myocardial injury via direct action by infecting cardiomyocytes after identifying ACE2 receptor and by indirect injury via inflammatory storm inducing immune response and oxygen supply imbalance (Zheng et al. 2020a, b; Fletcher-Sandersjöö and Bellander 2020). Thrombosis risk factors involve the stasis, endothelial injury, and a hypercoagulable state via inflammation leading to a decrease in fibrinolysis process, activation of the tissue factor pathway, and NETosis together known as Virchow’s triad including lifestyle and clinical factors such as obesity, immobility, pregnancy, and dehydration, surgery (Zheng et al. 2020a, b; Page and Ariëns 2021). Inflammation, complement activation, and coagulation cascade have an interplay role in initiating cardiovascular complications. C3a and C5-b are thought to be involved in platelet activation, enhanced plasma and cellular TF expression (Fletcher-Sandersjöö and Bellander 2020). In response to inflammation, the NETosis process involves releasing neutrophil extracellular traps (NETs) from neutrophils, releasing neutrophil granule contents and condensed chromatin. Release of polyphosphate leading platelet degranulation via direct viral myocardial and microvascular injury leading subendothelium and collagen exposure, initiating platelet stimulation and possible contact pathway activation. The tissue factor pathway leads to the cleavage of FVII to FVIIa. On the other hand, ACE2 SARS-CoV-2 interactions, the main receptor for SARS-CoV-2 entry, may also dysregulate the kallikrein/kinin system and virus, contributing to contact pathway activation also (Van Nieuwkoop 2020). Furthermore, the SARS-CoV-2 N1 gene has detected mRNA in the patient’s platelets sample. Viruses, namely influenza, are endocytosed by platelets results in C3 release and NETosis via TLR7. Similarly, SARS-CoV-2 RNA is also thought to react with platelets via TLR7 and TLR9 (Toll-like receptors) to stimulate leukocytes and release inflammatory cytokine (Bautista-Vargas et al. 2020). NETs, an important part of the immune response, effectively trap the bacteria, fungi, and viruses and interact with the coagulation and inflammatory cascades, potentiating the activation of platelets, endothelial cells, and FXII activation to the activation of endothelial cells increased resistance of clots to fibrinolysis. Therefore, here, it has seemed that NETosis might play a key role in connecting the release of inflammatory mediators, activation of platelets and endothelium, clot formation via resistance to fibrinolysis. Thrombus deposition further leads to cardiovascular complications. Another lethal mechanism suggesting fibrinolysis derangement and multiorgan failure is the disseminated intravascular coagulation. DIC has been suggested as a critical sign of health deterioration in COVID patients leading to cardiovascular complications. DIC pathophysiology involves the endothelial cell activation, leukocytes and platelets activation, fibrin deposition leading to inflammation and coagulopathy (Al-Ani et al. 2020; Page and Ariëns 2021; Gómez et al. 2021).
Furthermore, acute myocardial injury and myocarditis are the cardiovascular complications known to be associated with increased risk of severe condition and death in COVID-19 patients. It has been reported that there is a delay between the development of myocardial damage and symptom onset. Acute myocardial injury may be due to the virus binding receptor, i.e., ACE2 in the heart and virus-mediated lysis of cardiomyocytes. After SARS-CoV-2 binding with ACE2 receptors, the enzyme’s cardio protective effects may diminish or reduce due to the decreased activity of ACE2. Myocardial injury and inflammation interplay in COVID-19 patients have been suggested; the possible reason behind myocarditis in these patients, as both, could individually potentiate the observed pro-coagulative state through immune-thrombosis (Zheng et al. 2020a, b; Page and Ariëns 2021). Wenzel et al. in their study, evidenced the SARS-CoV-2 in endomyocardial biopsies of 2 patients with a previous infection with COVID-19 and suspected myocarditis (Wenzel et al. 2020). Cytokine storm due to inflammation leads to an increase in inflammatory cell migration and plaque infiltration, resulting in atherosclerosis, plaque instability, and arterial thrombus formation (Singh and Singh 2020). COVID-19 patients with vulnerable diseases such as hypertension and diabetes are more likely to develop atherosclerosis and myocarditis, requiring hospital admission (Sanyaolu et al. 2020; Zheng et al. 2020a, b; Page and Ariëns 2021).
The SARS-CoV-2 infection causes cardiac myocytes injury through myocardial infarction or myocarditis and may further lead to enhanced arrhythmia risk (Bhatla et al., 2020; Coromilas et al. 2021). Although it is difficult to conclude the underlying pathophysiology of arrhythmias in COVID-19, various studies evidenced some pathophysiological links. Globally several studies and surveys witnessed a spectrum of electrophysiology issues related to the COVID-19 and its therapies, mainly atrioventricular block, polymorphic ventricular tachycardia, and atrial fibrillation (Wang et al. 2020; Kochav et al. 2020). Another etiological factor for arrhythmia in COVID-19 patients includes right ventricular strain caused by pulmonary hypertension or embolism. Arrhythmia can also be produced by CD8 + T cells migrating into the heart and causing cardiac inflammation through cell-mediated cytotoxicity. This is caused mainly by lymphocyte overactivation due to cytokine storm, which further overproduces proinflammatory mediators, leading to a positive feedback loop of immunological activation and cardiac injury (Akkawi and Ghazal 2021; Coromilas et al. 2021). Coexisting electrolyte disarray, hypoxia, endogenous catecholamine adrenergic status, and the use of arrhythmogenic drugs such as azithromycin, hydroxychloroquine make it elusive to find the direct and indirect presence of COVID-19 on cardiac arrhythmias (Gopinathannair et al. 2020; Coromilas et al. 2021). Concerns about the danger of arrhythmia related to SARS-CoV-2 infection were raised after a 58% rise in out-of-hospital cardiac arrest in the Lombardy region of Italy during the first 40 days of the COVID-19 pandemic (Baldi et al. 2020). A major single-center study of 700 patients in the United States found that admission to the intensive care unit was associated with a tenfold increase in the incidence of arrhythmia (Bhatla et al., 2020). According to a case series reported by Liu et al., palpitations were recorded as the initial symptom in 7.3% SARS-CoV-2-positive patients admitted to tertiary hospitals in Hubei province in January 2020 (Liu et al. 2020). Moreover, patients with underlying CVD had higher troponin-T (TnT) levels, which led to more significant problems such as malignant arrhythmias and ventricular fibrillation (Akkawi and Ghazal 2021).
Therefore, underlying cardiovascular diseases such as hypertension, myocardial injury, arrhythmias, thrombosis, and diabetes mellitus are considered the risk factors for increased COVID-19 infection severity and increased mortality (Sanyaolu et al. 2020; Singh et al. 2020a, b, c; Zheng et al. 2020a, b; Driggin et al. 2020; Gómez et al. 2021; Page and Ariëns 2021). The above discussion addressed the comorbidities as risk factors for the progression to severe conditions or even death in COVID-19 patients.
COVID-19 and anosmia or ageusia
Gustatory and olfactory dysfunctions have developed as a symptom of COVID-19 rather than the associated symptoms such as dyspnea, fever, sore throat, myalgia, dry cough, fatigue, and headache. Gustatory dysfunctions vary between ageusia, dysgeusia, hypogeusia, and phantogeusia and are taste abnormalities. These signs’ development may be abrupt or slow (Harikrishnan 2020; Cetinkaya 2020). In many situations, they may occur related to inflammation, autoimmune illness, malignancy, nerve damage, psychological problems, radiotherapy, hormone imbalance, aging, etc. In viral upper respiratory infections and continuing to follow an influenza-like illness, taste loss has also been observed to result often (Harikrishnan 2020). When investigators first recognized smell loss as a COVID-19 symptom, they were concerned that the virus was infecting the nose’s odor-sensing neurons which send signals to the brain’s olfactory bulb, and also that the virus could access the brain as a result. Post-mortem studies of people with COVID-19 have shown that the virus rarely enters the brain (Marshall 2021). The molecular pathways contributing to gustatory dysfunction are not yet clear, but the target cells for the entrance of the virus are ACE2 receptors, as described above. This suggests that as the findings of the single-cell RNA-sequence profiles show the expression of ACE2 in the epithelial cells of an oral cavity and is greater in the tongue epithelium than those of the buccal and gingival tissues, the oral cavity is the route of the SARS-CoV-2 entry. In addition, tongue epithelium involvement can lead to taste disturbances (Harikrishnan 2020). A team of researchers in Italy found that taste and smell loss can occur simultaneously as a rise in the blood levels of an inflammation-signaling molecule called interleukin-6 (IL-6) (South et al. 2020). In 214 COVID-19 patients hospitalized in Wuhan, hyposmia and hypogeusia were present in 5.1% and 5.6% of cases, respectively (Han et al. 2020). Detailed neurological symptoms of COVID-19-hospitalized patients are reported in the analysis: 5% of patients had hyposmia and about 3% of patients had a lowered taste sensation as well. In 76%, the onset of anosmia was sudden and steadily decreased the sense of smell in 60% of patients before the questionnaire was completed. 83% of these patients also had a lowered taste sensation associated with anosmia (Cetinkaya 2020). There are more records of gustatory dysfunction in European studies, although there is an absence of such symptoms in Asian studies. The median recovery time from ageusia and anosmia was 7 days, but in some cases, most patients recover in 3 weeks (Lee et al. 2020). Women are particularly vulnerable to developing these chemo-sensitive symptoms relative to males. These gender-based variations can be related to the variance in the inflammatory reaction phase.
COVID-19 and Parkinson’s disease
SARS-CoV-2 is a significant concern for individuals with Parkinson’s disease (PD) worldwide as infections are the common etiological factor of exacerbating PD symptoms. Fatal infections like COVID-19 may have a direct detrimental effect on PD motor symptoms. First COVID-19 neurological complication was reported in patients of PD (Fearon and Fasano 2021). Many reports suggest, for those diagnosed with PD, the possibility of being infected is not greater. Still, the likelihood of developing complications of COVID-19 and severe respiratory disease tends to be high or at least in later stages of PD. In addition, due to COVID-19, PD’s non-motor and motor symptoms will aggravate significantly (Bloem et al. 2021). It is very well known that the typical non-motor characteristics of PD are ageusia and anosmia (Sharma et al. 2021a, b). Therefore, during COVID-19, the episode of hypogeusia and hyposmia could be a slight “hit” for the potential development of PD symptoms (Boika 2020). Preclinical animal studies have confirmed that SARS-CoV or MERS-CoV in transgenic mice is over-expressed by human ACE2 or human dipeptidyl peptidase 4, respectively, after intranasal inoculation. SARS-CoV-2 infection is more likely in cells that express ACE2 and TMPRSS2 (Transmembrane Serine Protease) through its spike glycoprotein S. Glycoprotein S must be cleaved by TMPRSS2 for this interaction to occur. Chen et al. looked into ACE2 expression using data from brain transcriptome databases. The SARS-CoV-2 receptor was found in both excitatory and inhibitory neurons and in oligodendrocytes, astrocytes, and the SN and brain ventricles (Sulzer et al. 2020; Salles-Gándara et al. 2020). It is also observed that mice overexpress the viral receptor, but these reports do not show normal infection routes. MERS-CoV and SARS-CoV may invade in the brain and transmit via olfactory nerves to the nuclei of CNS, including the brain and thalamus (Bloem et al. 2021). PD patients with COVID-19 infection are found to be obese, have Vitamin D deficiency and underlying COPD compared with PD patients who do not get COVID-19 infection. As known, obesity and lung diseases are the well-explained risk factors in other populations, but the inverse relationship with Vitamin D supports the hypothesis that Vitamin D deficiency may lead to COVID-19 susceptibility (de Siqueira et al. 2020; Alqahtani et al. 2020; Mitchell 2020). Vitamin D therapy was protective against PD and COVID-19, both as hypovitaminosis is very common in PD patients (Ilie et al. 2020; Ding et al. 2013; Hribar et al. 2020). However, more research is needed to develop pathophysiological links. Neuronal α-synuclein expressions in the peripheral nervous system block viral CNS invasion and replication of RNA viruses in the nervous system (Grewal et al., 2019). In a preclinical study, α-synuclein knockout mice showed B and T cell deficiencies. Loss of α-synuclein neuronal function may play an important role in the pathogenesis of PD as α-synuclein is abundant in synaptic clefts and plays an important role in the recycling of synaptic vesicles synaptic transmission (Salles-Gándara et al. 2020). The physiological function of α-synuclein exhibits the role in the immune cell recruitment and protection against proinflammatory responses to other infections. Therefore, it was concluded that α-synuclein overexpression in PD patients might block the process of neuroinvasion of SARS-CoV-2 (Fearon and Fasano 2021). Coronavirus may also enter the CNS through the lymphatic and hematogenous path. Still, it is unlikely at the initial disease stage because the SARS-CoV particles in the human post-mortem brain tissue have not been found in the non-neuronal cells (Bloem et al. 2021). The disintegration of the blood–brain barrier (BBB) caused by a cytokine storm linked with a peripheral infection could be a mechanism for SARS-CoV-2-RNA involvement in the CNS. The proinflammatory cytokines linked to SARS-CoV-2 infection and inflammation such as interleukin-1β and TNF-α mediate the breakdown of BBB. Equivalent to neurodegenerative conditions that lead to the invasion of immune cells and viral particles resulting in Encephalitis, such a breakdown might be either long-term (Sulzer et al. 2020). Moreover, PD is most often associated with several comorbidities, specifically to CVD; so for this cause, it has been judged that PD patients might fall into the high-risk group to contract severe COVID-19 infection, particularly the old age group (Sorbera et al. 2021). In addition, the reported association between PD and age and cardiovascular comorbidities creates an indirect risk. There is another direct link between PD patients with pre-existing respiratory dysfunction and a large severity of COVID-19 disease. Respiratory muscle weakness and aberrant posture, resulting in respiratory muscle rigidity and insufficient respiratory excursions, lead to ventilator failure in advanced stages of PD (Scorza et al. 2018; Baille et al. 2019; Sorbera et al. 2021). If one has Parkinson’s, then he/she can take the vaccine as soon as possible as it has high benefits. Two COVID-19 vaccines—mRNA-1273 (Moderna) and BNT162b2 (Pfizer/BioNTech) are currently in supply across the U.S. with some others in development across the globe (Bloem et al. 2021).
COVID-19 and anakusis
Many viral infections can cause hearing loss. Virus-induced sensorineural hearing loss (SNHL) may be acquired or congenital, either unilateral or bilateral, but a conductive and mixed hearing loss could be seen soon after viral infections (Mustafa 2020). Occasionally, hearing recovery occurs spontaneously after these infections. The first study on the relationship between COVID-19 and hearing loss was from Thailand, where there is a coincidence of neurosensory hearing loss in one old female case (Sriwijitalai & Wiwanitkit 2020). Another case study from the UK of a 45-year-old asthmatic patient documented hearing loss when COVID-19 was being treated. On the 10th day of COVID-19, the patient was hospitalized and intubated in the ICU for 30 days. In addition, anemia and pulmonary hypertension have complicated the situation. After undergoing plasma therapy, intravenous steroids, and redelivering patient’s condition improved, and the patient reported left-side tinnitus and unexpected hearing loss a week after the extubation from ICU. COVID-19 signs are tinnitus, vertigo, and abrupt hearing loss, as the ACE2 receptors have recently been found to be expressed in the middle ear epithelial cells of an animal model. In addition, the hearing could be impaired by the reaction of an immune response to the infection (Trecca et al. 2020). In a small study from Israel, 16 patients were asked to verify if the COVID-19 damages the auditory system. Half were positive (case group), whereas half were not positive for COVID-19 (control group). While using measures of otoacoustic emissions (OAE) and auditory brain stem response (ABR), researchers reported no difference in the signs of auditory damage in the two groups (Gérard et al. 2020).
Many theories have been proposed about COVID-19’s pathophysiology and the mechanisms that may lead to this atypical disease manifestations. An impairment in the temporal lobe’s auditory center due to viral pathophysiology mediated by angiotensin-converting enzyme 2 (ACE2), involvement of the inner ear’s microvasculature or the auditory center, or a peripheral injury (to the hair cells of the cochlea) due to the virus’s neurotropism are all possible etiologies. Another possibility is that COVID-19 causes alterations in the microvasculature, either through thrombi or emboli, which could result in ischemic lesions in the inner ear or auditory center. SARS-CoV-2 infection has been linked to a variety of thrombosis-related problems, both venous and arterial, resulting in a multiorgan systemic syndrome. This virus appears to produce endotheliitis in the temporal lobe’s hearing center, the cochlear nerve, and cochlear tissues (Cure and Cure 2020; Harenberg et al. 2020; Saniasiaya 2021).
COVID-19, and age, weight, and blood group
Many studies have demonstrated increased age and overweight as risk factors for COVID-19. Other studies also show that type A blood group can increase the risk of spreading the disease and carry a risk of serious illness. The explanation for this is not apparent (Ad’hiah et al. 2020). Some reports suggest that the blood epitopes could promote the entry of the virus into the human host. A case–control analysis was performed with a control group of 901 blood donors and 1014 Iraqi COVID-19-hospitalized patients. In nasopharyngeal swabs, the diagnosis was made by detecting coronavirus RNA. It was then observed that type A blood group can be related with a risk of developing Nasopharyngeal COVID-19 swabs, particularly in males (Ad’hiah et al. 2020). It is further proposed that when contaminated with COVID-19, better outcomes are observed in blood type O carriers. Hypertensive patients have been shown to usually have a high ACE/ANGII axis, where ACE controls the angiotensin II levels in RAAS. It has been reported that Chinese patients with essential HTN and ABO blood group is associated with ACE inhibitor-induced cough and ACE activity. The GATC haplotype of the 4 ABO gene polymorphisms (rs12683493, rs8176740, rs8176746, and rs495828), which are prevalent in patients with non-O blood types and is correlated with ACE operation. Lower levels of ACE2 are observed in blood type O carriers, which could provide an alternative reason for the blood group O COVID-19 carriers’ milder manifestations (Abdel Massih et al. 2020; Latz et al. 2020).
Since comorbidities are increasingly common as people get older, the elderly may face more severe problems related to COVID-19. Due to the apparent pathophysiological alterations that characterize the respiratory system, aging itself has been closely linked to poor outcomes. Immunosenescence is a well-known characteristic of aging. Disruption of both the innate and adaptive branches of the immune system has been described as people get older. Furthermore, the aged produce inflammatory mediators and cytokines continuously, a process characterized as inflammation (Aw et al. 2007; Longobardi et al. 2016). Abnormal ciliary function and ciliary ultrastructural abnormalities in elderly persons may threaten virus SARS-CoV-2 particle clearance (Ho et al. 2001; Simonnet et al. 2020).
Adults with excess weight are at even greater risk during the COVID-19 pandemic. Obesity raises the risk of COVID-19-related severe illness. Overweight people may also be at a higher risk. Obesity may triple the risk of COVID-19 infection-related hospitalization. It has been related to a weakened immune system. Obesity leads to reduces lung capacity and reserve, making breathing more difficult. A study of COVID-19 cases found that increasing BMI increases the likelihood of hospitalization, intensive care unit admission, invasive mechanical ventilation, and mortality (Tanaka et al. 2001; Simonnet et al. 2020).
COVID-19 and sleep apnea
Obstructive sleep apnea (OS A), which affects nearly 1 billion people worldwide and has a prevalence of nearly 50% in certain countries, is one of the common pulmonary diseases (Miller & Cappuccio 2020). COVID-19 exposure may increase OSA risk in those who already have the condition since they both involve and influence the respiratory system. As a result, for the prevention and control of COVID-19 susceptibility in OSA patients, extra help and a novel therapeutic approach should be introduced (Bonsignore et al. 2019). The OSA and COVID-19 relationship has been hypothesized but not yet confirmed. Popular risk factors shared with OSA for COVID-19 mortality include increased age, HTN, CV disease, pulmonary disease, and DM (Miller & Cappuccio 2020). Compared to a similar age group receiving treatment in a broad, geographically, and socio-economically diverse healthcare system, OSA patients faced an estimated eightfold greater risk of COVID-19 infections. In 28% of the 21 patients with coronavirus infection who reported to their ICU, a report found that OSA was present. OSA was associated with a high hospitalization risk and the risk of experiencing respiratory failure is nearly doubled in COVID-19 patients (Maas et al. 2020). The cellular entry of SARS-CoV-2 depends on ACE2, and ACE2 activity in OSA is increased (Hoffmann et al. 2020). There is a strong link between angiotensin-converting enzyme receptor II (ACE II) and hypertension in OSA (Koyama et al. 2009). Furthermore, the cleavage of S-protein with the ACE2 receptor by the transmembrane protease serine 2 (TMPRSS2) elucidates their entry. It regulates hypertension in mild to moderate early diagnosed OSA patients (Heurich et al. 2014). However, enhanced underlying inflammation can be of particular significance in obese individuals as it can potentially lead to worsening hypoxemia and the cytokine storm that occurs in pneumonia patients with COVID-19 and those with subsequent multiorgan failure. The findings of a French study of 124 patients with COVID suggested that overweight (BMI > 30 kg/m2) was a risk factor for intrusive mechanical ventilation, regardless of age, DM and HTN, but no OSA frequency was registered. Recent research has shown that melatonin, the sleep hormone, can benefit COVID-19 therapy as it can minimize inflammation, oxidative stress, and immune response. OSA was separately correlated with higher hsCRP and lipids and lower vitamin D in a study of 176 children. In addition, there is growing evidence to indicate that the potential risk for CVD could be vitamin D deficiency (Miller and Cappuccio 2020). In suppressing inflammatory markers and harmful reactive oxygen species (ROS), vitamin D may play a significant role and may stimulate the development of protective endothelial nitric oxide (NO). COVID-19 is distributed by droplets mainly. Continuous positive airway pressure (CPAP) is a droplet-generating process that can potentially intensify the virus’s spread (Miller and Cappuccio 2020; Tang et al. 2021).
Emerging SARS-CoV-2 variants
Viruses mutate, and it is essential to keep a focus on whether the virus behavior is changing. Sometimes different strains emerge and then disappear, and in some other instances, new variants emerge and stay. New variants often arise and other times new variants appear and remain (Koyama et al. 2020). The virus is named after the structure which are crown-like on their surface. Scientists monitor changes in the viruses, including changes to the surface spikes of the viruses (Giovanetti et al. 2021). These variants are seen to spread more quickly and easily than other variants. Studies have also suggested that antibodies generated via vaccination with currently authorized vaccines recognize new variants (Rubin and Longo 2020). The globally circulating multiple variants that cause COVID-19 are mentioned in Table 1. A new mutation (E484K) has also been seen among these variants that help the mutant viruses to evade the antibodies (Rubin and Longo 2020; Giovanetti et al. 2021).
Table 1.
Multiple variants that cause COVID-19 globally (Rubin and Longo 2020; Giovanetti et al. 2021)
| Country | Variant name | Disease severity | Detected | Mutation |
|---|---|---|---|---|
| U.K | B.1.1.7 | Increased death severity | December 2020 | 23 mutations |
| South Africa | B.1.351 | No evidence | October 2020 | Multiple |
| Brazil | P.1 | Less vulnerable to antibodies | January 2020 | 17 mutations |
| India | B.1.671.2 (Delta) | More severe illnesses | October 2020 | 13 mutations |
Some of the probable effects of the variants are the following:
The ability of faster spread among the population.
The ability to cause milder or severe illness in humans.
The tendency of such viral diagnostic tests to resist detection.
Reduced therapeutic susceptibility.
Capability to evade immunity induced naturally or by the vaccine.
To restrict the spread of the virus and protect public health, strict and enhanced adherence to public health prevention measures, such as vaccines, social distancing, masks usage, manual hygiene maintenance, isolation, and quarantine, is critical (Giovanetti et al. 2021).
COVID-19 vaccines
Vaccines are a critical new instrument in the fight against COVID-19, and so many vaccines are extremely encouraging and successful. Scientists from around the world work together and innovate as quickly as possible to bring tests, treatments and vaccines that will cure lives and end this pandemic collectively. Different studies which are linked with COVID-19 and various comorbidities are mentioned in Table 2.
Table 2.
Different studies linked with COVID-19 and various comorbidities
| Study reference | Country name/study design | Number of patients | Major findings |
|---|---|---|---|
| Nishiga et al. (2020) | Multi-center cohort study in Wuhan, China | 191 patients | Cardiovascular disease (CVD) patients and risk factors such as diabetes, and HTN are at extreme risk of developing COVID-19 manifestations |
| Manolis et al. (2020) | US | 700 COVID patients | Prevalence of AF, cardiac arrest in COVID-19 patients |
| Gérard et al. (2020) | Israel | 16 patients (case: COVID positive and control: COVID-negative groups) | No difference in the signs of auditory damage in the two groups |
| Ad’hiah et al. (2020) | Iraq | 901 control blood group donors and 1104 COVID patients | Blood group A has an elevated the risk of developing COVID-19 |
| Wang et al. (2020) | Wuhan, China | 138 patients | CFR and mortality rate in patients with and without comorbid conditions were observed |
Pfizer-Bio N Tech COVID-19 vaccine
US Food and Drug Administration on December 11th, 2020, announced the first emergency use authorization (EUA) vaccine for preventing COVID-19. The US government has approved an emergency use permit for the Pfizer-Bio N tech COVID-19 Vaccine for delivery to individuals 16 years and above (Smetanová et al. 2020). Pfizer-Bio N Tech COVID-19 Vaccine side effects reported include fatigue, musculoskeletal pain, injection site pain, joint pain, injection site redness, nausea, and lymphadenopathy. A remote risk of causing a serious allergic reaction by the Pfizer-Bio N Tech COVID-19 Vaccine within several minutes to an hour of the vaccine dose. Difficulty in breathing, facial and throat swelling, rapid pulse, rashes all over the body, and dizziness can also be a sign of extreme allergic reaction (Smetanová et al. 2020). The Pfizer-Bio N Tech COVID-19 Vaccine will be given intramuscularly. The Pfizer-Bio N Tech COVID-19 Vaccine vaccination series is conducted in 2 doses which are to be given 3 weeks apart (Smetanová et al. 2020).
Moderna COVID-19 vaccine
This is the second FDA-approved COVID-19 prevention emergency vaccine. A modern vaccine can be split into the population of the United States for people 18 years and older through emergency use. The protective action against COVID-19 begins after the first dose within 14 days and is reportedly 92% effective. Moderna COVID-19 Vaccine is given intramuscularly. The vaccination series is given 2 doses and each dose 1 month apart (Dash et al. 2021). Reported side effects include reactions to the lymph nodes in the same arm of injections: discomfort, tenderness and swelling, swelling (hardness) and redness. General side effects are the following: weakness, headache, muscle pain, joint pain, chills, nausea and vomiting, and fever. A severe allergic reaction is typical of a dose of the vaccine within a few minutes to an hour and this can include—breathing problems, face and throat swelling, rapid pulse, poor rash around the body, dizziness, and weakness (Dash et al. 2021). The recommended use of Moderna Vaccine is at a schedule of 2 doses (100 mg, 0.5 ml each) 28 days apart. The interval between the doses may extend to 42 days (Dash et al. 2021).
COVAXIN
COVAXIN, the indigenous vaccine by Bharat Biotech, was developed with the Indian Council for Medical Research (ICMR) (Cui et al. 2019). The vaccine is engineered using a platform technology from Whole-Virion Inactivated Vero Cell. Inactivated vaccines would not replicate, so reverting and triggering pathological effects are impossible. They contain dead viruses which cannot infect people but can still instill a shielding response to infections in the immune system. Immune cells will still recognize the dead virus, leading to antibodies to the pandemic virus (Cui et al. 2019). COVAXIN contains immune potentiators that are applied to a vaccine in to increase and increase immunogenicity, also known as vaccine adjuvants. The vaccine is a 2-dose vaccine administered 28 days apart (Silveira et al. 2021). DCGI approval was given to the vaccine in July 2020 for Phase I and II of human clinical trials. For Phase 3 multicentre trial, all participants were given the first vaccine dose and currently are given the second vaccine/placebo dose. The occurrence of COVID-19 infection between the vaccinated group and placebo group is expected to be effective and to begin 2 weeks after the second dose. By the end of February 2021, the interim efficacy evaluation will be generated (Cui et al. 2019). COVAXIN for restricted emergency use in India by DCGI-CDSCO on 03 January 2021(Cui et al. 2019). Side effects observed were pain, swelling or itching, malaise, weakness, rashes, fever, nausea and vomiting. The factsheet warns about a severe allergic reaction that includes rashes all over the body, dyspnea, facial swelling, tachycardia, dizziness, and weakness (Cui et al. 2019).
Oxford-AstraZeneca vaccine—CoviShield
The local production of the Oxford-AstraZeneca vaccine is carried out by the Serum Institute of India (SII), the biggest producer of the world’s vaccines. It says more than 50 million doses are produced every month. The vaccine called CoviShield is a weakened form of a common cold virus (adenovirus). It is made to look like coronavirus—but it cannot cause a disease (Silveira et al. 2021). Injecting the vaccine to a patient stimulates the immune system to develop antibodies and encourages it to attack all coronavirus infections. The jab is given in two doses, 4 to 12 weeks apart. It can be stored safely and at temperatures between 2 °C and 8 °C and roughly the same as a household refrigerator. International Oxford-AstraZeneca clinical tests have shown that efficacy reaches 90% when the patient is administered a half and then a full dose. SII has indicated “very common” side effects that can affect over 1 person out of 10, including redness, itching, pain, swelling, warmth, fatigue, tenderness, itching, chills, nausea, headache, fever, and pain or muscle distress. SII has reported that it is normal for 1 in every 10 people to have side effects, such as injection lumps, vomiting, fever, flu symptoms such as sore throat, cough, runny nose, temperature, and chill (Chung et al. 2021). SII has suggested “uncommon” side effects that can impact up to one in every 100 individuals. These include dizziness, declining appetite, stomach pain, lymph nodes swollen, heavy sweating, itchy skin, or rash (Chung et al. 2021).
Sputnik V
Sputnik V is the first human adenoviral vector-based vaccine registered worldwide. In more than 25 countries, Sputnik V is now licensed. More than 31,000 volunteers participated in the latest clinical trial in Russia. The phase 3 clinical studies of Sputnik V are being carried out in India, Belarus, Venezuela, and UAE (Balakrishnan 2020). The vaccine has more than 90% effectiveness. Sputnik V efficacy is confirmed 91.6%, based on data of 19,866 volunteers who had received the initial doses of the vaccine. Weakness and muscle pain were reported in 1 in 7 volunteers who received Sputnik V vaccine dose (Bassetti et al. 2021). Vaccines with these dosage and common side effects are demonstrated in Table 3.
Table 3.
Dosage and effects of the COVID-19 vaccines (Cui et al. 2019; Smetanová et al. 2020; Mahase, 2020; Silveira et al. 2021; Balakrishnan, 2020; Bassetti et al. 2021; Chung et al. 2021; Dash et al. 2021)
| Vaccine | Allergic reaction | Side effects | Dose |
|---|---|---|---|
| Pfizer-Bio N Tech Covid-19 vaccine | Difficulty in breathing, rapid pulse, swelling of throat and face, bad rash, dizziness | Fatigue, musculoskeletal pain, nausea, joint pain, injection site pain and redness, lymphadenopathy | 2 doses were given 3 weeks apart |
| Moderna Covid-19 vaccine | Breathing problems, face and throat swelling, a bad rash, rapid pulse, dizziness and weakness | Lymphadenopathy, discomfort and tenderness in the same arm of injection, weakness, chills, joint pain, nausea, headache, fever, muscle pain, and vomiting | 2 doses (100 mg, 0.5 ml each) 28 days apart. Interval may extend to 42 days |
| COVAXIN | Tachycardia, breathing difficulty, swelling of face and throat, rashes, dizziness and weakness | Malaise, pain, swelling, itching, fever, weakness, rashes, nausea and vomiting | 2 doses given 28 days apart |
| Oxford-AstraZeneca vaccine—CoviShield | Rash, dizziness, and weakness | Redness, itching, pain, swelling, fatigue, nausea, muscle distress, headache, flu symptoms, injection lumps, anorexia | 2 doses given 4–12 weeks apart |
| Sputnik V | Rashes, convulsions, muscle weakness and breathing difficulties | Weakness and muscle pain, seizures, breathing difficulties and possible encephalomyelitis | Two shots based on two different viral vectors and given 21 days apart |
Other candidates for evaluating protection and efficacy are at various stages in India
ZyCov-Di
It is established by Zydus-Cadila from Ahmedabad. A vaccine is being produced in partnership with the US-based Dynavax and College of Medicine in Hyderabad, the first Indian private vaccine maker, Biological E (Silveira et al. 2021).
HGCO19
India’s first Pune-based Genovese mRNA vaccine, in partnership with Seattle-based Biotech Corporation, uses genetic code bits to induce an immune reaction (Silveira et al. 2021).
Bharat BioTech’s nasal vaccines
The Serum Institute of India and Novavax American Development company are developing their second vaccine (Silveira et al. 2021).
Antiviral treatment against COVID-19
Currently, no specific medicine is effective for the treatment of SARS-CoV-2. Antiviral drugs are being researched for the treatment of COVID-19 because SARS-CoV-2 replication shows many clinical symptoms. The antiviral drugs being investigated block viral entrance through the ACE2 receptor and the transmembrane serine protease 2 (TMPRSS2), including viral membrane fusion and endocytosis, the activity of the SARS-CoV-2-3-chymotrypsin-like protease. The treatment of COVID-19 entails developing algorithms that include drugs aimed at slowing disease progression by combating two distinct but intertwined processes which first includes the virus caused damage with antivirals and second damage caused by a dysregulated host response with drugs having immunomodulatory property (Soleimanpour and Yaghoubi 2021; Indari et al. 2021). Remdesivir (viral RNA polymerase inhibitor) is currently the only FDA-approved drug for COVID-19 treatment (Riva et al. 2020; Indari et al. 2021). Nowadays, drug repurposing is an effective technique for quickly identifying frontline arsenals to combat COVID-19. Because of the possible success of this method, some clinically approved medications have been repurposed as possible anti-SARS-CoV-2 agents, including antimalarials, antibiotics, corticosteroids and majorly antivirals (Indari et al. 2021; Peng et al. 2021). Antiviral drugs may have an impact against COVID-19 at the earliest stage, before the sickness advances to the hyperinflammatory state that can define the latter stages of the disease, including critical illness as viral replication, may be active at the earliest stages of COVID-19 infection (Riva et al. 2020; Indari et al. 2021). Therefore, it is important to recognize the role and impact of antiviral therapy in treating and managing mild to severe critical illness to develop a treatment for COVID-19 patients. Antiviral and antimicrobial drugs that are approved or under investigation for the treatment of SARS-CoV-2 has been listed below in table. The recommended dose is mentioned according to the data published in various articles (Table 4).
Table 4.
Recommended drug treatment strategies for COVID-19
| Prescribed drug | Recommended dose in COVID-19 | References |
|---|---|---|
| Remdesivir (an RNA polymerase inhibitor) |
Day1: 200 mg Day 2–5:100 mg IV (can be extended upto 10 days) |
Hung and Yuen (2020) |
| Favipiravir (an RNA polymerase inhibitor) | 2 × 1600 mg/day | Chen et al. (2020) |
| Ivermectin (opening of Glutamate-gated chloride channels) | IVM 0.2–0.6 mg/kg PO given as a single dose or as a once-daily dose for up to 5 days (In adults) | Yavuz and Ünal (2020) |
| Hydroxychloroquine or chloroquine/Azithromycin |
Hydroxychloroquine = 2 × 200 mg/day (orally) Chloroquine = 2 × 500 mg/day (orally) |
Indari et al. (2021) |
| Lopinavir/Ritonavir (an inhibitor of aspartate protease of human immunodeficiency virus) | 2 × 400 mg/day (orally) | Peng et al. (2021) |
| Ribavirin (inosine monophosphate dehydrogenase inhibitor + immunomodulatory action) | 2 × 400 mg (along with interferon-alpha 400 mg for 14 days | Peng et al. (2021) |
Conclusion and future perspectives
SARS-CoV-2 and CVD seem to interact in a particular aspect. Although COVID-19 is primarily a respiratory disorder, a significant number of COVID-19-infected patients with pre-existing CVD experience a new-onset heart dysfunction during the disease. High-quality data on the occurrence, prevalence, clinical manifestations, outcomes, and therapeutic modalities of CVD in the sense of COVID-19 must be presented in future studies. The diagnostic, therapeutic, and public health problems that combine these two diseases cannot be addressed without new knowledge. Hypertension has been observed among severely ill patients, our current knowledge has been altered through observational studies that show an association without identifying the cause. Critically ill COVID-19 patients also suffer from comorbidity, which may cause life-threatening VAs, including highly proarrhythmic electrolyte abnormalities (magnesemia, hypokalemia), labile autonomic control, fever, and systemic inflammatory disease. Especially in patients with acute myocardial injury, the cardiac rhythms prevalence is greater than that which has no myocardial damage. Among COVID-19-related myocardial injuries, etiologies include myocarditis, myocardial infarction, myocardial injuries related to sepsis and SARS-CoV-2 Cardiomyopathy which adds CV burden in patients. Moreover, the exact mechanisms are not known, SARS-CoV-2 is found to cause both COVID-19 and myocardial damage to host cells via ACE2. In SARS-CoV-2 and CVD patients with underlying infection, adverse prognoses may be present. A few vaccines have shown positive effects against COVID-19 infection and several better treatments have been investigated. Treatment options for various diseases linked with COVID-19 such as urolithiasis, Parkinson’s disease, sleep apnea, and obesity, have markedly changed during the pandemic. For emergency cases, if an alarming indication is present, emergency intervention should be provided and for non-emergency cases, informed treatment decisions should be made.
Acknowledgements
The authors are grateful to the Chitkara College of Pharmacy, Chitkara University, Rajpura, Patiala, Punjab, India for providing the necessary facilities to carry out the research work.
Author contributions
Conceptualization: conceived and designed the experiments: TGS. Analyzed the data: RK and SS. Wrote the manuscript: RK, SS, PS, and JR. Visualization: TGS, and SS. Editing of the manuscript: RK, SS, and TGS. Critically reviewed the article: TGS. Supervision: TGS. All the authors read and approved the final manuscript.
Funding
Nil.
Availability of data and material
Not applicable.
Declarations
Conflict of interest
There are no conflicts of interest.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abdel Massih AF, Mahrous R, Taha A, Saud A, Osman A, Kamel B, Yacoub E, Menshawey E, Ismail HA, Aita L, Dous M. The potential use of ABO blood group system for risk stratification of COVID-19. Med Hypotheses. 2020;145:110343. doi: 10.1016/j.mehy.2020.110343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ad’hiah AH, Abdullah MH, Alsudani MY, Shnawa RM, Al-Sa’ady AJ, Allami RH, Misha’al KI, Jassim IA, Taqi EA. Association between ABO blood groups and susceptibility to COVID-19: profile of age and gender in Iraqi patients. Egypt J Med Hum Genet. 2020;21:1. doi: 10.1186/s43042-019-0045-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad T, Rodriguez-Morales AJ. ) Emergence of COVID-19 (formerly 2019-novel Coronavirus): a new threat from China. Rev Panam Enferm Infecc. 2019;2(2):37–38. [Google Scholar]
- Akkawi AR, Ghazal M. COVID-19 and cardiac arrhythmias: a review of the literature. Cureus. 2021 doi: 10.7759/cureus.17797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Ani S, Chehade S, Lazo-Langner A. Thrombosis risk associated with COVID-19 infection. A scoping review. Thromb Res. 2020;192:152–160. doi: 10.1016/j.thromres.2020.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Sadeq DW, Nasrallah GK. The incidence of the novel coronavirus SARS-CoV-2 among asymptomatic patients: a systematic review. Int J Infect Dis. 2020;98:372–380. doi: 10.1016/j.ijid.2020.06.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alqahtani JS, Oyelade T, Aldhahir AM, Alghamdi SM, Almehmadi M, Alqahtani AS, Hurst JR. Prevalence, severity and mortality associated with COPD and smoking in patients with COVID-19: a rapid systematic review and meta-analysis. PLoS One. 2020;15:e0233147. doi: 10.1371/journal.pone.0233147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonucci M, Recupero SM, Marzio V, De Dominicis M, Pinto F, Foschi N, Di Gianfrancesco L, Bassi P, Ragonese M. The impact of COVID-19 outbreak on urolithiasis emergency department admissions, hospitalizations and clinical management in central Italy: a multicentric analysis. Actas Urol Esp. 2020;44:611–616. doi: 10.1016/j.acuroe.2020.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apicella M, Campopiano MC, Mantuano M, Mazoni L, Coppelli A, Del Prato S. COVID-19 in people with diabetes: understanding the reasons for worse outcomes. Lancet Diabetes Endocrinol. 2020 doi: 10.1016/S2213-8587(20)30238-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aw D, Silva AB, Palmer DB. Immunosenescence: emerging challenges for an ageing population. Immunology. 2007;120:435–446. doi: 10.1111/j.1365-2567.2007.02555.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babapoor-Farrokhran S, Gill D, Walker J, Rasekhi RT, Bozorgnia B, Amanullah A. Myocardial injury and COVID-19: possible mechanisms. Life Sci. 2020;253:117723. doi: 10.1016/j.lfs.2020.117723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baille G, Chenivesse C, Perez T, Machuron F, Dujardin K, Devos D, Moreau C. Dyspnea: an underestimated symptom in Parkinson’s disease. Parkinsonism RelatDisord. 2019;60:162–166. doi: 10.1016/j.parkreldis.2018.09.001. [DOI] [PubMed] [Google Scholar]
- Balakrishnan VS. The arrival of Sputnik V. Lancet Infect Dis. 2020;20:1128. doi: 10.1016/S1473-3099(20)30709-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldi E, Sechi GM, Mare C, Canevari F, Brancaglione A, Primi R, Klersy C, Palo A, Contri E, Ronchi V, Beretta G. Out-of-hospital cardiac arrest during the Covid-19 outbreak in Italy. N Engl J Med. 2020;383:496–498. doi: 10.1056/NEJMc2010418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee AB, Singh TG, Prashar A. BCG vaccine, a ray of hope in treating Severe Acute Respiratory Syndrome (SARS) Infect Disord Drug Targets. 2021;21(5):e270421186935. doi: 10.2174/1871526520666201016150501. [DOI] [PubMed] [Google Scholar]
- Bansal M. Cardiovascular disease and COVID-19. Diabetes Metab Syndr: Clin Res Rev. 2020;14:247–250. doi: 10.1016/j.dsx.2020.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassetti M, Corcione S, Dettori S, Lombardi A, Lupia T, Vena A, De Rosa FG, Gori A, Giacobbe DR. Antiviral treatment selection for SARS-CoV-2 pneumonia. Expert Rev Respir Med. 2021;22:1–8. doi: 10.1080/17476348.2021.1927719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bautista-Vargas M, Bonilla-Abadía F, Cañas CA. Potential role for tissue factor in the pathogenesis of hypercoagulability associated with in COVID-19. J Thromb Thrombolysis. 2020;50:479–483. doi: 10.1007/s11239-020-02172-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatla A, Mayer MM, Adusumalli S, Hyman MC, Oh E, Tierney A, Deo R. COVID-19 and cardiac arrhythmias. Heart Rhythm. 2020;17:1439–1444. doi: 10.1016/j.hrthm.2020.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloem BR, Trenkwalder C, Sanchez-Ferro A, Kalia LV, Alcalay R, Chiang HL, Kang UJ, Goetz C, Brundin P, Papa SM. COVID-19 vaccination for persons with Parkinson’s disease: light at the end of the tunnel? Parkinson's Dis. 2021;28:1–5. doi: 10.3233/JPD-212573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boika AV. A post-COVID-19 Parkinsonism in the future? J Mov Disord. 2020 doi: 10.1002/mds.28117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonsignore MR, Baiamonte P, Mazzuca E, Castrogiovanni A, Marrone O. Obstructive sleep apnea and comorbidities: a dangerous liaison. Multidiscip Respir Med. 2019;14:1–12. doi: 10.1186/s40248-019-0172-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cetinkaya EA. Coincidence of COVID-19 infection and smell—taste perception disorders. J Craniofac Surg. 2020 doi: 10.1097/SCS.0000000000006601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen PL, Lee NY, Cia CT, Ko WC, Hsueh PR. A review of treatment of coronavirus disease 2019 (COVID-19): therapeutic repurposing and unmet clinical needs. Front Pharmacol. 2020 doi: 10.3389/fphar.2020.584956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung JY, Thone MN, Kwon YJ. COVID-19 vaccines: the status and perspectives in delivery points of view. Adv Drug Deliv Rev. 2021;170:1–25. doi: 10.1016/j.addr.2020.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clerkin KJ, Fried JA, Raikhelkar J, Sayer G, Griffin JM, Masoumi A, Uriel N. COVID-19 and cardiovascular disease. Circulation. 2020;141:1648–1655. doi: 10.1161/CIRCULATIONAHA.120.046941. [DOI] [PubMed] [Google Scholar]
- Cook TM. The importance of hypertension as a risk factor for severe illness and mortality in COVID-19. Anaesthesia. 2020;75:976. doi: 10.1111/anae.15103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coromilas EJ, Kochav S, Goldenthal I, Biviano A, Garan H, Goldbarg S, Kim JH, Yeo I, Tracy C, Ayanian S, Akar J. Worldwide survey of COVID-19—associated arrhythmias. Circ: Arrhythm Electrophysiol. 2021;14:e009458. doi: 10.1161/CIRCEP.120.008719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cristelo C, Azevedo C, Marques JM, Nunes R, Sarmento B. SARS-CoV-2 and diabetes: new challenges for the disease. Diabetes Res Clin Pract. 2020;164:108228. doi: 10.1016/j.diabres.2020.108228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui S, Chen S, Li X, Liu S, Wang F. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J Thromb Haemost. 2020;18(6):1421–1424. doi: 10.1111/jth.14830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui J, Li F, Shi ZL. Origin and evolution of pathogenic coronaviruses. Nat Rev Microbiol. 2019;17(3):181–92. doi: 10.1038/s41579-018-0118-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui X, Zhao Z, Zhang T, Guo W, Guo W, Zheng J, Zhang J, Dong C, Na R, Zheng L, Li W, Liu Z, Ma J, Wang J, He S, Xu Y, Si P, Shen Y, Cai C. A systematic review and meta-analysis of children with coronavirus disease 2019 (COVID-19) J Med Virol. 2021;93:1057–1069. doi: 10.1002/jmv.26398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cure E, Cure MC. Comment on “Hearing loss and COVID-19: a note”. Am J Otolaryngol. 2020;41:102513. doi: 10.1016/j.amjoto.2020.102513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dash GC, Subhadra S, Turuk J, Parai D, Rath S, Sabat J, Rout UK, Kanungo S, Choudhary HR, Nanda RR, Pattnaik M, Pati S, Bhattacharya D. Breakthrough SARS-CoV-2 infections among BBV-152 (COVAXIN®) and AZD1222 (COVISHIELDTM ) recipients: report from eastern state of India. J Med Virol. 2021 doi: 10.1002/jmv.27382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Siqueira JV, Almeida LG, Zica BO, Brum IB, Barceló A, de Siqueira Galil AG. Impact of obesity on hospitalizations and mortality, due to COVID-19: a systematic review. Obes Res Clin Pract. 2020;14(5):398–403. doi: 10.1016/j.orcp.2020.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deepika AS, Kaur R, Singh TG, Singh M, Satija S, Singh R. Prescribing pattern of antihypertensive drugs in a tertiary care hospital: a review. Plant Arch. 2019;19(2):1311–1316. [Google Scholar]
- Di Gennaro F, Pizzol D, Marotta C, Antunes M, Racalbuto V, Veronese N, Smith L. Coronavirus diseases (COVID-19) current status and future perspectives: a narrative review. Int J Environ Res Public Health. 2020;17:2690. doi: 10.3390/ijerph17082690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding H, Dhima K, Lockhart KC, Locascio JJ, Hoesing AN, Duong K, Trisini-Lipsanopoulos A, Hayes MT, Sohur US, Wills A-M, Mollenhauer B, Flaherty AW, Hung AY, Mejia N, Khurana V, Gomperts SN, Selkoe DJ, Schwarzschild MA, Schlossmacher MG, Hyman BT, Sudarsky LR, Growdon JH, Scherzer CR. Unrecognized vitamin D3 deficiency is common in Parkinson disease: Harvard biomarker study. Neurology. 2013;81:1531–1537. doi: 10.1212/wnl.0b013e3182a95818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly CA, Ghani AC, Leung GM, Hedley AJ, Fraser C, Riley S, Anderson RM. Epidemiological determinants of spread of causal agent of severe acute respiratory syndrome in Hong Kong. The Lancet. 2003;361:1761–1766. doi: 10.1016/S0140-6736(03)13410-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driggin E, Madhavan MV, Bikdeli B, Chuich T, Laracy J, Biondi-Zoccai G, Parikh SA. Cardiovascular considerations for patients, health care workers, and health systems during the COVID-19 pandemic. J Am Coll Cardiol. 2020;75:2352–2371. doi: 10.1016/j.jacc.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang L, Karakiulakis G, Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir Med. 2020;8:e21. doi: 10.1016/S2213-2600(20)30116-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearon C, Fasano A. Parkinson’s disease and the COVID-19 pandemic. J Parkinsons Dis. 2021 doi: 10.3233/JPD-202320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez C, Rysä J, Almgren P, Nilsson J, Engström G, Orho-Melander M, Melander O. Plasma levels of the proprotein convertase furin and incidence of diabetes and mortality. J Intern Med. 2018;284:377–387. doi: 10.1111/joim.12783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher-Sandersjöö A, Bellander BM. Is COVID-19 associated thrombosis caused by overactivation of the complement cascade? A literature review. Thromb Res. 2020;194:36–41. doi: 10.1016/j.thromres.2020.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gérard C, Maggipinto G, Minon JM. COVID-19 and ABO blood group: another viewpoint. Br J Haematol. 2020;190:e93–e94. doi: 10.1111/bjh.16884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovanetti M, Benedetti F, Campisi G, Ciccozzi A, Fabris S, Ceccarelli G, Tambone V, Caruso A, Angeletti S, Zella D, Ciccozzi M. Evolution patterns of SARS-CoV-2: snapshot on its genome variants. BiochemBiophys Res Commun. 2021;538:88–91. doi: 10.1016/j.bbrc.2020.10.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez CA, Sun CK, Tsai I, Chang YP, Lin MC, Hung IY, Hung KC. Mortality and risk factors associated with pulmonary embolism in coronavirus disease 2019 patients: a systematic review and meta-analysis. Sci Rep. 2021;11:1–13. doi: 10.1038/s41598-021-95512-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopinathannair R, Merchant FM, Lakkireddy DR, Etheridge SP, Feigofsky S, Han JK, Russo AM. COVID-19 and cardiac arrhythmias: a global perspective on arrhythmia characteristics and management strategies. J Interv Card Electrophysiol. 2020;59:329–336. doi: 10.1007/s10840-020-00789-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grewal AK, Singh N, Singh TG. Effects of resveratrol postconditioning on cerebral ischemia in mice: role of the sirtuin-1 pathway. Can J Physiol Pharmacol. 2019;97(11):1094–1101. doi: 10.1139/cjpp-2019-0188. [DOI] [PubMed] [Google Scholar]
- Gupta A, Madhavan MV, Sehgal K, Nair N, Mahajan S, Sehrawat TS, Bikdeli B, Ahluwalia N, Ausiello JC, Wan EY, Freedberg DE. Extrapulmonary manifestations of COVID-19. Nat Med. 2020;26:1017–1032. doi: 10.1038/s41591-020-0968-3. [DOI] [PubMed] [Google Scholar]
- Guzik TJ, Mohiddin SA, Dimarco A, Patel V, Savvatis K, Marelli-Berg FM, McInnes IB. COVID-19 and the cardiovascular system: implications for risk assessment, diagnosis, and treatment options. Cardiovasc Res. 2020;116:1666–1687. doi: 10.1093/cvr/cvaa106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han AY, Mukdad L, Long JL, Lopez IA. Anosmia in COVID-19: mechanisms and significance. Chem Senses. 2020;45:423–428. doi: 10.1093/chemse/bjaa040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanff TC, Mohareb AM, Giri J, Cohen JB, Chirinos JA. Thrombosis in COVID-19. Am J Hematol. 2020;95:1578–1589. doi: 10.1002/ajh.25982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harenberg J, Jonas JB, Trecca EM. A liaison between sudden sensorineural hearing loss and SARS-CoV-2 infection. Thromb Haemost. 2020;120:1237–1239. doi: 10.1055/s-0040-1714370. [DOI] [PubMed] [Google Scholar]
- Harikrishnan P. Gustatory dysfunction as an early symptom in COVID-19 screening. J Craniofac Surg. 2020 doi: 10.1097/SCS.0000000000006797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heurich A, Hofmann-Winkler H, Gierer S, Liepold T, Jahn O, Pöhlmann S. TMPRSS2 and ADAM17 cleave ACE2 differentially and only proteolysis by TMPRSS2 augments entry driven by the severe acute respiratory syndrome coronavirus spike protein. J Virol. 2014;88:1293–1307. doi: 10.1128/JVI.02202-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho JC, Chan KN, Hu WH, Lam WK, Zheng L, Tipoe GL, Tsang KW. The effect of aging on nasal mucociliary clearance, beat frequency, and ultrastructure of respiratory cilia. Am J Respir Crit Care Med. 2001;163:983–988. doi: 10.1164/ajrccm.163.4.9909121. [DOI] [PubMed] [Google Scholar]
- Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, Müller MA. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hribar CA, Cobbold PH, Church FC. Potential role of vitamin D in the elderly to resist COVID-19 and to slow progression of Parkinson’s disease. Brain Sci. 2020;10:284. doi: 10.3390/brainsci10050284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung IF, Yuen KY. Early triple antiviral therapy for COVID-19–Authors’ reply. The Lancet. 2020;396:1488. doi: 10.1016/S0140-6736(20)32268-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilie PC, Stefanescu S, Smith L. The role of vitamin D in the prevention of coronavirus disease 2019 infection and mortality. Aging Clin Exp Res. 2020;32:1195–1198. doi: 10.1007/s40520-020-01570-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indari O, Jakhmola S, Manivannan E, Jha HC. An update on antiviral therapy against SARS-CoV-2: how far have we come? Front Pharmacol. 2021;12:133. doi: 10.3389/fphar.2021.632677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji T, Liu Z, Wang G, Guo X, Lai C, Chen H, Zhou Q. Detection of COVID-19: A review of the current literature and future perspectives. Biosens Bioelectron. 2020;166:112455. doi: 10.1016/j.bios.2020.112455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn JS, McIntosh K. History and recent advances in coronavirus discovery. Pediatr Infect Dis J. 2005;24:S223–S227. doi: 10.1097/01.inf.0000188166.17324.60. [DOI] [PubMed] [Google Scholar]
- Kanwal A, Agarwala A, Martin LW, Handberg EM, Yang E (2020) COVID-19 and hypertension: what we know and don't know. Am Coll Cardiol
- Khan A. Prevalence, pathophysiological mechanisms and factors affecting urolithiasis. Int Urol Nephrol. 2018;50:799–806. doi: 10.1007/s11255-018-1849-2. [DOI] [PubMed] [Google Scholar]
- Kochav SM, Coromilas E, Nalbandian A, Ranard LS, Gupta A, Chung MK. Cardiac arrhythmias in COVID-19 infection. Circ Arrhythm Electrophysiol. 2020;13:e008719. doi: 10.1161/CIRCEP.120.008719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama RG, Drager LF, Lorenzi-Filho G, Cintra FD, Pereira AC, Poyares D, Pedrazzoli M. Reciprocal interactions of obstructive sleep apnea and hypertension associated with ACE I/D polymorphism in males. Sleep Med. 2009;10:1107–1111. doi: 10.1016/j.sleep.2008.12.012. [DOI] [PubMed] [Google Scholar]
- Koyama T, Platt D, Parida L. Variant analysis of SARS-CoV-2 genomes. Bull World Health Organ. 2020;98:495–504. doi: 10.2471/BLT.20.253591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai CC, Shih TP, Ko WC, Tang HJ, Hsueh PR. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): the epidemic and the challenges. Int J Antimicrob Agents. 2020;55:105924. doi: 10.1016/j.ijantimicag.2020.105924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latz CA, DeCarlo C, Boitano L, Png CM, Patell R, Conrad MF, Eagleton M, Dua A. Blood type and outcomes in patients with COVID-19. Ann Hematol. 2020;99:2113–2118. doi: 10.1007/s00277-020-04169-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Min P, Lee S, Kim SW. Prevalence and duration of acute loss of smell or taste in COVID-19 patients. J Korean Med Sci. 2020 doi: 10.3346/jkms.2020.35.e174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim S, Bae JH, Kwon HS, Nauck MA. COVID-19 and diabetes mellitus: from pathophysiology to clinical management. Nat Rev Endocrinol. 2021;17(1):11–30. doi: 10.1038/s41574-020-00435-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu K, Fang YY, Deng Y, Liu W, Wang MF, Ma JP, Liu HG. Clinical characteristics of novel coronavirus cases in tertiary hospitals in Hubei Province. Chin Med J. 2020;133:1025. doi: 10.1097/CM9.0000000000000744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long B, Brady WJ, Koyfman A, Gottlieb M. Cardiovascular complications in COVID-19. Am J Emerg Med. 2020;38:1504–1507. doi: 10.1016/j.ajem.2020.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longobardi L, Di Giorgio A, Perrotta F, Costigliola A, Cerqua FS, Cioffi G, Stefanelli F. Bronchial asthma in the elderly patient. J Gerontol Geriatr. 2016;64:55–65. [Google Scholar]
- Maas MB, Kim M, Malkani RG, Abbott SM, Zee PC. Obstructive sleep apnea and risk of COVID-19 infection, hospitalization and respiratory failure. Sleep Breath. 2020;29:1–3. doi: 10.1007/s11325-020-02203-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahase E. Covid-19: moderna applies for US and EU approval as vaccine trial reports 94.1% efficacy. BMJ. 2020;371:m4709. doi: 10.1136/bmj.m4709. [DOI] [PubMed] [Google Scholar]
- Manolis AS, Manolis AA, Manolis TA, Apostolopoulos EJ, Papatheou D, Melita H. COVID-19 infection and cardiac arrhythmias. Trends Cardiovasc Med. 2020 doi: 10.1016/j.tcm.2020.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall M. COVID’s toll on smell and taste: what scientists do and don’t know. Nature. 2021;589:342–343. doi: 10.1038/d41586-021-00055-6. [DOI] [PubMed] [Google Scholar]
- Miller MA, Cappuccio FP. A systematic review of COVID-19 and obstructive sleep apnoea. Sleep Med Rev. 2020;8:101382. doi: 10.1016/j.smrv.2020.101382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell F. Vitamin-D and COVID-19: do deficient risk a poorer outcome? Lancet Diabetes Endocrinol. 2020;8:570. doi: 10.1016/S2213-8587(20)30183-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustafa MW. Audiological profile of asymptomatic Covid-19 PCR-positive cases. Am J Otolaryngol. 2020;41:102483. doi: 10.1016/j.amjoto.2020.102483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagashima K, Wege H, Meyermann R, Ter Meulen V. Corona virus induced subacute demyelinating encephalomyelitis in rats: a morphological analysis. Acta Neuropathol. 1978;44:63–70. doi: 10.1007/BF00691641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni W, Yang X, Yang D, Bao J, Li R, Xiao Y, Gao Z. Role of angiotensin-converting enzyme 2 (ACE2) in COVID-19. J Crit Care. 2020;24:1–10. doi: 10.1186/s13054-020-03120-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiga M, Wang DW, Han Y, Lewis DB, Wu JC. COVID-19 and cardiovascular disease: from basic mechanisms to clinical perspectives. Nat Rev Cardiol. 2020;17:543–558. doi: 10.1038/s41569-020-0413-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page EM, Ariëns RA. Mechanisms of thrombosis and cardiovascular complications in COVID-19. Thromb Res. 2021;200:1–8. doi: 10.1016/j.thromres.2021.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal M, Berhanu G, Desalegn C, Kandi V. Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2): an update. Cureus. 2020 doi: 10.7759/cureus.7423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paramasivam A, Priyadharsini JV, Raghunandhakumar S, Elumalai P. A novel COVID-19 and its effects on cardiovascular disease. Hypertens Res. 2020;43:729–730. doi: 10.1038/s41440-020-0461-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng L, Shen L, Xu J, Tian X, Liu F, Wang J, Tian G, Yang J, Zhou L. Prioritizing antiviral drugs against SARS-CoV-2 by integrating viral complete genome sequences and drug chemical structures. Sci Rep. 2021;11:1–1. doi: 10.1038/s41598-021-83737-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen E, Koopmans M, Go U, Hamer DH, Petrosillo N, Castelli F, Simonsen L. Comparing SARS-CoV-2 with SARS-CoV and influenza pandemics. Lancet Infect Dis. 2020 doi: 10.1016/S1473-3099(20)30484-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raheem AA, Alowidah I, Soliman M, Haresy M, Almozeni A, Althagafi S, Almousa M, Alturki M. Urolithiasis treatment options during COVID-19 pandemic: review of current recommendations and triage systems. Afr J Urol. 2020;26:1–7. doi: 10.1186/s12301-020-00085-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehman M, Gondal A, Rehman NU. Atypical manifestation of COVID-19-induced myocarditis. Cureus. 2020 doi: 10.7759/cureus.8685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rostami M, Mansouritorghabeh H. D-dimer level in COVID-19 infection: a systematic review. Expert Rev Hematol. 2020;13:1265–1275. doi: 10.1080/17474086.2020.1831383. [DOI] [PubMed] [Google Scholar]
- Rubin EJ, Longo DL. SARS-CoV-2 Vaccination—an ounce (actually, much less) of prevention. N Engl J Med. 2020;383:2677–2678. doi: 10.1056/NEJMe2034717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salles-Gándara P, Rojas-Fernandez A, Salinas-Rebolledo C, Milan-Sole A. The potential role of SARS-COV-2 in the pathogenesis of Parkinson’s disease. Front Neurol. 2020;11:1044. doi: 10.3389/fneur.2020.01044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samudrala PK, Kumar P, Choudhary K, Thakur N, Wadekar GS, Dayaramani R, Alexander A. Virology, pathogenesis, diagnosis and in-line treatment of COVID-19. Eur J Pharmacol. 2020;883:173375. doi: 10.1016/j.ejphar.2020.173375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saniasiaya J. Hearing loss in SARS-CoV-2: what do we know? Ear Nose Throat J. 2021;100:152S–154S. doi: 10.1177/0145561320946902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santesmasses D, Castro JP, Zenin AA, Shindyapina AV, Gerashchenko MV, Zhang B, Gladyshev VN. COVID-19 is an emergent disease of aging. Aging Cell. 2020;19:e13230. doi: 10.1111/acel.13230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanyaolu A, Okorie C, Marinkovic A, Patidar R, Younis K, Desai P, Altaf M. Comorbidity and its impact on patients with COVID-19. SN Compr Clin Med. 2020 doi: 10.1007/s42399-020-00363-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scorza FA, Fiorini AC, Scorza CA, Finsterer J. Cardiac abnormalities in Parkinson’s disease and Parkinsonism. J ClinNeurosci. 2018;53:1–5. doi: 10.1016/j.jocn.2018.04.031. [DOI] [PubMed] [Google Scholar]
- Sharma VK, Singh TG. Chronic stress and diabetes mellitus: interwoven pathologies. Curr Diabetes Rev. 2020;16(6):546–556. doi: 10.2174/1573399815666191111152248. [DOI] [PubMed] [Google Scholar]
- Sharma VK, Singh TG. Insulin resistance and bioenergetic manifestations: targets and approaches in Alzheimer’s disease. Life Sci. 2020;262:118401. doi: 10.1016/j.lfs.2020.118401. [DOI] [PubMed] [Google Scholar]
- Sharma VK, Singh TG. Navigating Alzheimer’s disease via chronic stress: the role of glucocorticoids. Curr Drug Targets. 2020;21(5):433–444. doi: 10.2174/1389450120666191017114735. [DOI] [PubMed] [Google Scholar]
- Sharma A, Khan H, Singh TG, Grewal AK, Najda A, Kawecka-Radomska M, Kamel M, Altyar AE, Del-Daim MM. Pharmacological modulation of ubiquitin-proteasome pathways in oncogenic signaling. Int J Mol Sci. 2021;22(21):11971. doi: 10.3390/ijms222111971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma T, Kaur D, Grewal AK, Singh TG. Therapies modulating insulin resistance in Parkinson’s disease: a cross talk. Neurosci Lett. 2021;749:135754. doi: 10.1016/j.neulet.2021.135754. [DOI] [PubMed] [Google Scholar]
- Sher L. COVID-19, anxiety, sleep disturbances and suicide. Sleep Med. 2020;70:124. doi: 10.1016/j.sleep.2020.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silveira MM, Moreira GMSG, Mendonça M. DNA vaccines against COVID-19: perspectives and challenges. Life Sci. 2021;267:118919. doi: 10.1016/j.lfs.2020.118919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonnet A, Chetboun M, Poissy J, Raverdy V, Noulette J, Duhamel A, Verkindt H. High prevalence of obesity in severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) requiring invasive mechanical ventilation. Obesity. 2020;28:1195–1199. doi: 10.1002/oby.22831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S, Singh TG. Role of nuclear factor Kappa B (NF-κB) signalling in neurodegenerative diseases: an mechanistic approach. Curr Neuropharmacol. 2020;18(10):918–935. doi: 10.2174/1570159X18666200207120949.PMID:32031074;PMCID:PMC7709146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh AK, Gupta R, Ghosh A, Misra A. Diabetes in COVID-19: Prevalence, pathophysiology, prognosis and practical considerations. Diabetes Metab Syndr: Clin Res Rev. 2020;14:303–310. doi: 10.1016/j.dsx.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R, Rao HK, Singh TG. Advanced glycated end products (ages) in diabetes and its complications: an insight. Plant Arch. 2020;20(1):3838–3841. [Google Scholar]
- Singh TG, Sharma R, Kaur A, Dhiman S, Singh R. Evaluation of renoprotective potential of Ficus religiosa in attenuation of diabetic nephropathy in rats. Obes Med. 2020;19:100268. doi: 10.1016/j.obmed.2020.100268. [DOI] [Google Scholar]
- Singh R, Rao HK, Singh TG. Comparison of safety and efficacy of pregabalin, duloxetine and their combination with epalrestat in diabetic neuropathy: a prospective, double-blind, randomized, controlled Trial. J Appl Pharm Sci. 2021;11:071–79. [Google Scholar]
- Smetanová J, Střížová Z, Bartůňková J, Milota T. Principles and new perspectives in the vaccination against SARS-CoV-2 virus. Cas Lek Cesk. 2020;159:298–302. [PubMed] [Google Scholar]
- Soleimanpour S, Yaghoubi A. COVID-19 vaccine: where are we now and where should we go? Expert Rev Vaccines. 2021;20:23–44. doi: 10.1080/14760584.2021.1875824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorbera C, Brigandì A, Cimino V, Bonanno L, Ciurleo R, Bramanti P, Marino S. The impact of SARS-COV2 infection on people in residential care with Parkinson Disease or parkinsonisms: clinical case series study. PLoS One. 2021;16:e0251313. doi: 10.1371/journal.pone.0251313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- South AM, Diz DI, Chappell MC. COVID-19, ACE2, and the cardiovascular consequences. Am J Physiol Heart Circ Physiol. 2020;318:H1084–H1090. doi: 10.1152/ajpheart.00217.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava K. Association between COVID-19 and cardiovascular disease. Int J Cardiol Heart Vasc. 2020 doi: 10.1016/j.ijcha.2020.100583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sriwijitalai W, Wiwanitkit V. Hearing loss and COVID-19: a note. Am J Otolaryngol. 2020 doi: 10.1016/j.amjoto.2020.102473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramaniam A, Elsayed RM. Does obesity increase the morbidity and mortality in COVID-19 patients. evidence based clinical review. Sch J App Med Sci. 2021;2:248–252. doi: 10.36347/sjams.2021.v09i02.014. [DOI] [Google Scholar]
- Sulzer D, Antonini A, Leta V, Nordvig A, Smeyne RJ, Goldman JE, Al-Dalahmah O, Zecca L, Sette A, Bubacco L, Meucci O. COVID-19 and possible links with Parkinson’s disease and parkinsonism: from bench to bedside. NPJ Parkinsons Dis. 2020;6:1. doi: 10.1038/s41531-020-00123-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syed MA, Al Nuaimi AS, Nasrallah GK, Althani AA, Yassine HM, Zainel A, AAbdulmalik MA. Epidemiology of SARS-CoV2 in Qatar’s primary care population aged 10 years and above. BMC Infect Dis. 2021;21:1–11. doi: 10.1186/s12879-021-06251-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadic M, Cuspidi C, Mancia G, Dell’Oro R, Grassi G. COVID-19, hypertension and cardiovascular diseases: should we change the therapy? Pharmacol Res Commun. 2020;158:104906. doi: 10.1016/j.phrs.2020.104906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka SI, Isoda F, Ishihara Y, Kimura M, Yamakawa T. T lymphopaenia in relation to body mass index and TNF-α in human obesity: adequate weight reduction can be corrective. Clin Endocrinol. 2001;54:347–354. doi: 10.1046/j.1365-2265.2001.1139cn2155.x. [DOI] [PubMed] [Google Scholar]
- Tang JW, Tambyah PA, Hui DS. Emergence of a new SARS-CoV-2 variant in the UK. J Infect. 2021;82:e27–e28. doi: 10.1016/j.jinf.2020.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tefik T, Guven S, Villa L, Gokce MI, Kallidonis P, Petkova K, Kiremit MC, Sonmez MG, De Lorenzis E, Eryildirim B, Sarica K. Urolithiasis practice patterns following the COVID-19 pandemic: overview from the EULIS collaborative research working group. Eur Urol Suppl. 2020 doi: 10.1016/j.eururo.2020.04.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thapa K, Verma N, Singh TG, Kaur Grewal A, Kanojia N, Rani L. COVID-19-associated acute respiratory distress syndrome (CARDS): mechanistic insights on therapeutic intervention and emerging trends. Int Immunopharmacol. 2021;101(Pt A):108328. doi: 10.1016/j.intimp.2021.108328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tison GH, Avram R, Kuhar P, Abreau S, Marcus GM, Pletcher MJ, Olgin JE. Worldwide effect of COVID-19 on physical activity: a descriptive study. Ann Intern Med. 2020;173:767–770. doi: 10.7326/M20-2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasoni D, Italia L, Adamo M, Inciardi RM, Lombardi CM, Solomo SD, Metra M. COVID-19 and heart failure: from infection to inflammation and angiotensin II stimulation. Searching for evidence from a new disease. Eur J Heart Fail. 2020;22:957–966. doi: 10.1002/ejhf.1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trecca EMC, Gelardi M, Cassano M. COVID-19 and hearing difficulties. Am J Otolaryngol. 2020;41:102496. doi: 10.1016/j.amjoto.2020.102496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsatsakis A, Calina D, Falzone L, Petrakis D, Mitrut R, Siokas V, Docea AO. SARS-CoV-2 pathophysiology and its clinical implications: an integrative overview of the pharmacotherapeutic management of COVID-19. Food ChemToxicol. 2020;146:111769. doi: 10.1016/j.fct.2020.111769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Türk C, Petřík A, Sarica K, Seitz C, Skolarikos A, Straub M, Knoll T. EAU guidelines on diagnosis and conservative management of urolithiasis. Eur Urol. 2016;69(Suppl.1):468–474. doi: 10.1016/j.eururo.2015.07.040. [DOI] [PubMed] [Google Scholar]
- Unudurthi SD, Luthra P, Bose RJ, McCarthy J, Kontaridis MI. Cardiac inflammation in COVID-19: lessons from heart failure. Life Sci. 2020 doi: 10.1016/j.lfs.2020.118482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Nieuwkoop C. COVID-19 associated pulmonary thrombosis. Thromb Res. 2020;191:151. doi: 10.1016/j.thromres.2020.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vellingiri B, Jayaramayya K, Iyer M, Narayanasamy A, Govindasamy V, Giridharan B, Subramaniam MD. COVID-19: a promising cure for the global panic. Sci Total Environ. 2020;725:138277. doi: 10.1016/j.scitotenv.2020.138277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vishwakarma S, Singh S, Singh TG. Pharmacological modulation of cytokines correlating neuroinflammatory cascades in epileptogenesis. Mol Biol Rep. 2021 doi: 10.1007/s11033-021-06896-8. [DOI] [PubMed] [Google Scholar]
- Walls AC, Park YJ, Tortorici MA, Wall A, McGuire AT, Veesler D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell. 2020;181:281–292. doi: 10.1016/j.cell.2020.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Peng Z. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenzel P, Kopp S, Göbel S, Jansen T, Geyer M, Hahn F, Münzel T. Evidence of SARS-CoV-2 mRNA in endomyocardial biopsies of patients with clinically suspected myocarditis tested negative for COVID-19 in nasopharyngeal swab. Cardiovasc Res. 2020;116:1661–1663. doi: 10.1093/cvr/cvaa160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- www.Clinicaltrials.gov.in
- Yavuz S, Ünal S. Antiviral treatment of COVID-19. Turk J Med Sci. 2020;21(50):611–619. doi: 10.3906/sag-2004-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng YY, Ma YT, Zhang JY, Xie X. COVID-19 and the cardiovascular system. Nat Rev Cardiol. 2020;17:259–260. doi: 10.1038/s41569-020-0360-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Z, Peng F, Xu B, Zhao J, Liu H, Peng J, Tang W. Risk factors of critical & mortal COVID-19 cases: a systematic literature review and meta-analysis. J Infect. 2020;81:e16–e25. doi: 10.1016/j.jinf.2020.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Cao B. Clinical course and risk factors for mortality of adult in patients with COVID-19 in Wuhan, China: a retrospective cohort study. The Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.