Fig. 4. Primary human BCCs use E-selectin to enter a dormant sinusoidal perivascular niche in the BM.
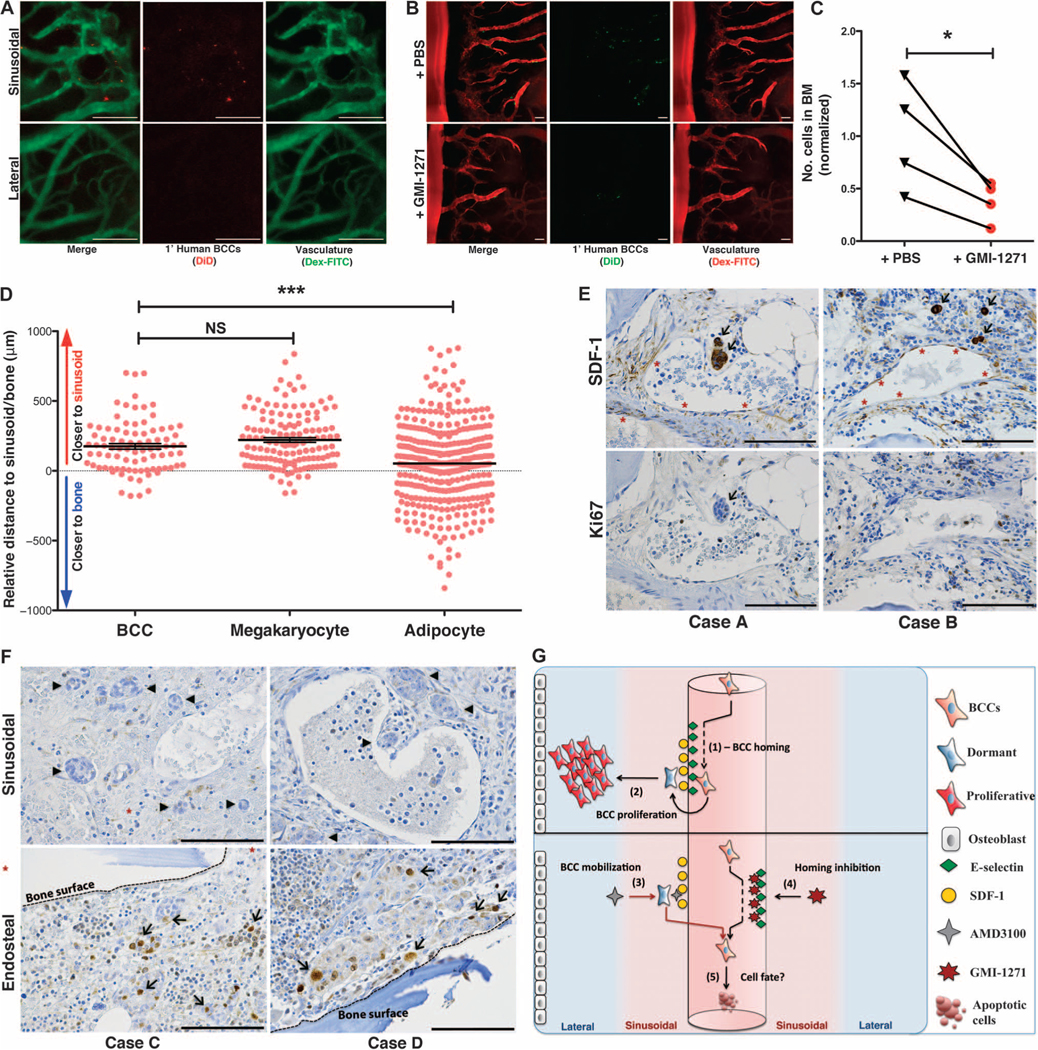
(A) Primary patient BCCs were isolated from surgical resection tissue and engrafted in mice. Intravital calvarial imaging revealed that primary cells home specifically to sinusoidal vasculature (n = 4). Representative images are shown. (B and C) Primary human BCCs were engrafted ± GMI-1271, and BM homing was quantified at 20 hours. Representative images are shown. Homing was decreased almost threefold in GMI-1271–treated mice (*P = 0.0393, n = 4; paired, two-way t test). (D) Histologic sections of BM biopsies from patients with micrometastatic involvement of breast cancer were obtained. The distance of each individual micrometastasis from the nearest sinusoid or bone spicule was measured by hand by a hematopathologist. Distance measurements for megakaryocytes, which are intimately associated with the sinusoidal vasculature, and for adipocytes, which are randomly scattered in bone, were also performed. Similar to megakaryocytes, breast cancer micrometastases were identified near sinusoids, with relative distance measurements that were significantly different from randomly distributed adipocytes [***P = 0.0002, data from three separate patient samples (BCCs, n = 86; megakaryocytes, n = 136; adipocytes, n = 348); unpaired, two-way t test]. (E) Immunohistochemical staining of BM biopsies from micrometastatic disease patients shows that BCCs (arrows) adjacent to SDF-1+ vasculature (asterisks) are Ki67−. (F) BM biopsy samples from patients with macrometastatic disease were stained for Ki67. Perisinusoidal (asterisks) BCCs were Ki67− (arrowheads), but Ki67+ BCCs (arrows) could be identified near bony spicules (dashed lines). (G) Cartoon model of BCC homing (1) and retention and proliferation (2) mechanisms in BM niches. Forced mobilization of dormant cells (3) combined with simultaneous blockade of BM reentry (4) could cause apoptosis or chemosensitize cells deprived of supportive stroma (5). Scale bars, 100 μm.
