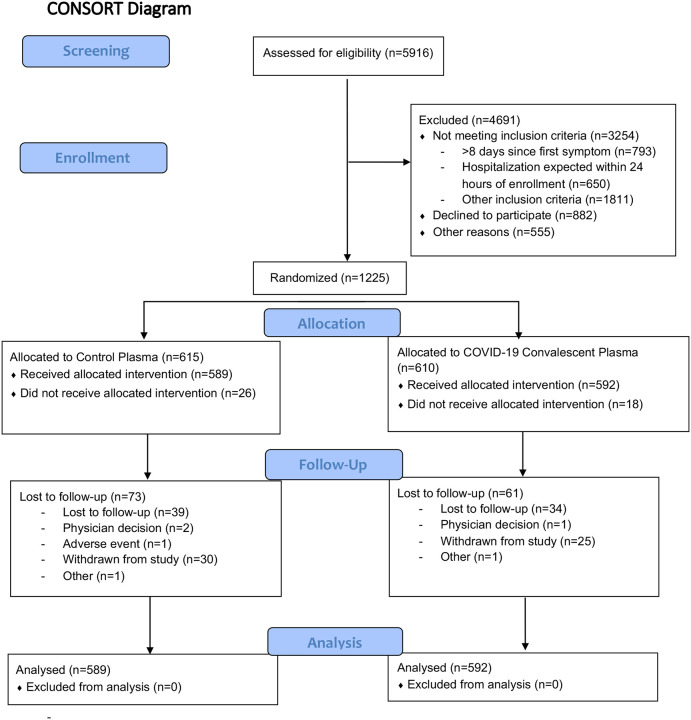
Figure 1. Enrollment, Randomization and Treatment Populations.
Potential participants who a diagnostic test positive for SARS-CoV-2 and < 8 days of COVID-19 were assessed both for eligibility by study personnel and by investigators to confirm that they were safe for outpatient management. Participants may have had >1 reason for exclusion from the trial. The intention-to-treat population included all randomized participants and the modified-ITT excluded randomized participants who did not receive assigned trial product.