Significance Statement
It is unknown whether low levels of lead exposure, such as those commonly encountered in drinking water systems, have adverse effects. Using data from patients initiating dialysis in the United States over the last 15 years, the authors found those living in cities with higher lead levels in the drinking water supply had significantly lower hemoglobin concentrations and more erythropoietin stimulating agent use. These associations were observed at lead levels significantly below those that the Environmental Protection Agency mandates as actionable. Whether such levels of lead exposure similarly associate with other lead-related diseases will require further study. The authors’ analysis suggests that for patients with kidney disease, there may be no safe amount of lead in drinking water.
Keywords: lead toxicity, anemia, environmental risk factors, disparities, racial and ethnic disparities
Visual Abstract
Abstract
Background
Although patients with kidney disease may be particularly susceptible to the adverse health effects associated with lead exposure, whether levels of lead found commonly in drinking water are associated with adverse outcomes in patients with ESKD is not known.
Methods
To investigate associations of lead in community water systems with hemoglobin concentrations and erythropoietin stimulating agent (ESA) use among incident patients with ESKD, we merged data from the Environmental Protection Agency (EPA) Safe Drinking Water Information System (documenting average 90th percentile lead concentrations in community water systems during 5 years before dialysis initiation, according to city of residence) with patient-level data from the United States Renal Data System.
Results
Among 597,968 patients initiating dialysis in the United States in 2005 through 2017, those in cities with detectable lead levels in community water had significantly lower pre-ESKD hemoglobin concentrations and more ESA use per 0.01 mg/L increase in 90th percentile water lead. Findings were similar for the 208,912 patients with data from the first month of ESKD therapy, with lower hemoglobin and higher ESA use per 0.01 mg/L higher lead concentration. These associations were observed at lead levels below the EPA threshold (0.015 mg/L) that mandates regulatory action. We also observed environmental inequities, finding significantly higher water lead levels and slower declines over time among Black versus White patients.
Conclusions
This first nationwide analysis linking EPA water supply records to patient data shows that even low levels of lead that are commonly encountered in community water systems throughout the United States are associated with lower hemoglobin levels and higher ESA use among patients with advanced kidney disease.
Although workplace contamination and ingestion of lead-based paint are known sources of lead exposure,1,2 the effect of more modest exposures, as occurs with levels commonly found in drinking water in the United States, is unknown. Lead is not naturally found in water, but enters the drinking supply primarily through the corrosion of lead pipes, faucets, and fixtures, and less frequently through contamination of groundwater systems.3 The Environmental Protection Agency (EPA) mandates surveillance of community water systems and regulatory action when the 90th percentile of samples exceeds 0.015 mg/L, known as an actionable level. However, no amount of lead in water is considered safe, and despite significant reductions in water contamination over the last three decades, most water systems do not uniformly meet the EPA’s Maximum Contaminant Level Goal of zero.4
Although children are known be at increased risk from lead exposure, unique complications of CKD confer similar susceptibility.5–10 Metabolic conditions highly prevalent in CKD, such as hypocalcemia, iron deficiency, and malnutrition, increase the proportion of ingested lead that is absorbed across the gastrointestinal tract.11–13 Simultaneously, patients with CKD less effectively excrete lead, culminating in circulating levels that are many fold higher than patients with normal renal function.14–16
Along with neurologic,17,18 cardiovascular,19–21 and endocrine complications,22,23 lead has important hematologic effects, interfering with heme biosynthesis, increasing red cell destruction24 and reducing gastrointestinal iron absorption.25,26 Lead toxicity has been associated with lower hemoglobin levels across a wide range of epidemiologic studies.27–31 Accordingly, to determine whether lead concentrations commonly found in modern community water systems across the United States have a myelosuppressive effect, we merged water system regulatory compliance data cataloged in the EPA’s Safe Drinking Water Information System (SDWIS) with patient-level data in the United States Renal Data System (USRDS). We examined the association of community water lead levels with hemoglobin concentrations and the frequency of erythropoietin stimulating agent (ESA) utilization before ESKD onset, and during the first month of dialysis therapy, among patients initiating dialysis between 2005 and 2017. In addition, given increasing awareness of environmental inequities in the United States, we examined whether there were racial and socioeconomic differences in the levels of lead exposure in community water.
Methods
Study Population
Using data from the USRDS, the national registry of patients with ESKD, we identified all incident patients with ESKD who initiated dialysis between January 1, 2005 and December 31, 2017. For each patient, we identified the city of residence, which was defined by a single zip code of residence ≥6 months before dialysis initiation, as defined by the first service date. We used data from the ESKD Medical Evidence Report, a standardized form that the Centers for Medicare and Medicaid Services requires for the registration of all incident patients with ESKD, to ascertain demographic information, comorbidities, pre-ESKD laboratory measurements, including serum creatinine and hemoglobin, and pre-ESKD ESA use. We used data in CROWNWeb, a Centers for Medicare and Medicaid Services data management system that all Medicare-certified dialysis facilities use to supply clinical information about prevalent patients on dialysis, to ascertain hemoglobin and ESA use during the first month of dialysis. CROWNWeb began to accept data in June 2012.
To estimate community water lead exposure, we used the SDWIS Federal Data Warehouse, which includes basic information and lead testing reports for all public community water systems in the United States, including the city or county served, number of people served, and the type of system (residential, transient, nontransient).32 We identified 30,759 community water systems that provided a service on a year-round basis and that defined their geographic service area by city, and examined 201,023 lead reports between January 1, 2000 and December 31, 2019 (Supplemental Figure 1). Water systems from eight states that did not describe geographic service area by city were excluded, as were those with implausible lead levels (>100-fold more than the EPA-actionable level, >1.5 mg/L, n=60). We linked the USRDS and SDWIS datasets by city and state to create a complete dataset of 597,968 incident patients with ESKD residing in 9566 cities serviced by 21,113 water systems. CROWNWeb data were available for 208,912 patients.
Primary Exposure Ascertainment
Federal guidelines mandate nonrandom household water sampling on the basis of the size of each water system and its previous lead concentrations, ranging from five to 100 samples per water system over 6-month to 3-year intervals.33 If ≥10% of these samples exceed the EPA’s actionable level of 0.015 mg/L, the water system must take corrective action and increase the frequency of testing. The lead level at the 90th percentile of water samples is reported in the SDIWS, according to the dates of water surveillance. We generated an annual lead level for each water system by matching the lead results to each year within the sampling period. Because repeated measures of lead occur in each surveillance period, particularly for water systems with elevated lead concentrations, we averaged the maximum reported lead level per year of all water systems serving a city, weighted for the number of individuals served by each water system, to derive an annual citywide lead level. Given a predictable annual decline in GFR of 2–3 ml/min, we calculated the average of citywide levels during the 5 years preceding dialysis initiation, because this period of acute deterioration would reflect heighted susceptibility to lead exposure.
Outcome Definitions
The primary outcomes were pre-ESKD hemoglobin concentration (recorded up to 45 days before dialysis initiation) and pre-ESKD ESA use, as recorded on the ESKD Medical Evidence Report. To account for the effect of pre-ESKD ESA use on hemoglobin, we also examined corrected hemoglobin concentration, which was set by subtracting a fixed decrement of 2 g/dl from the observed hemoglobin concentration in ESA users.34,35 Secondarily, we examined uncorrected and corrected hemoglobin concentration and ESA use during the first month of dialysis; we set corrected hemoglobin concentration analogously.
Covariates
Patient pre-ESKD characteristics included age, sex, race (white, Black, Asian, other/unknown), body mass index, eGFR (as calculated by the Modification in Diet in Renal Disease four-factor equation), insurance, and employment status, all at the time of ESKD onset, and the presence of diabetes mellitus, heart failure, hypertension, cancer, or tobacco use at any time during the 10 years before ESKD onset. Levels of air quality, as estimated by measures of fine particulate matter between 2003 and 2011 in outdoor air, and available in the Wide-ranging ONline Data for Epidemiologic Research online database, were included at the county level.36
Statistical Analyses
We summarized patient characteristics, with strata defined by breakpoints of the standard level of detection (<0.001 mg/L), and at 25%, 50%, and 100% of the EPA-actionable lead level (0.015 mg/L). We used generalized estimating equations, including a normally distributed dependent factor, all patient and socioeconomic characteristics as independent factors, and a working correlation matrix, to examine the adjusted association of citywide lead levels with hemoglobin and corrected hemoglobin concentrations before dialysis initiation and during the first month of dialysis. We explored progressive models of adjustment. Given marked changes in the management of anemia of CKD over the last 15 years, model 1 includes adjustment for year of ESKD onset. Model 2 adds the patient characteristics of age, sex, race, comorbidities, body mass index, and eGFR at ESKD onset. To account for socioeconomic determinants of health, model 3 adds patient employment and health insurance status at time of onset. Finally, to account for the environmental influence of air pollution on hemoglobin concentrations, model 4 (i.e., the primary model) adds county-level estimates of fine particulate air pollution. We likewise used generalized estimating equations, using the same incremental models above, to estimate the adjusted difference in ESA use (prevalence) before dialysis initiation and during the first month of dialysis. Despite the binary nature of the outcome, we used an identity link function, thereby constructing a linear probability regression model, so associations with outcomes could be readily interpreted on an absolute scale.
In all models, we assessed two parameterizations of lead levels1: in five categories defined by breakpoints of <0.001 mg/L and at 25%, 50%, and 100% of the EPA-actionable lead level2; as a cubic polynomial, with lead levels winsorized at 0.1 mg/L to limit the influence of outlying values on the shape of the association. In all regressions, we clustered by the combination of dialysis facility and year of dialysis initiation.
To examine for racial or socioeconomic determinants of lead exposure, we tested for differences in mean water lead levels across categories of race, insurance status, and employment status, and in counties with higher proportions of pediatric lead toxicity (as determined by the 75th and 90th percentiles of the National Childhood Blood Lead Surveillance Data 2012 estimates).36 We estimated changes in lead concentration per calendar year of ESKD incidence, by race, and tested whether the adjusted linear trends in lead concentration differed between Black and White patients. To examine for the possibility of stratum-specific misclassification, we used multiplicative interaction terms to test whether the adjusted change in hemoglobin concentrations per 0.01 mg/L higher 90th percentile community water lead differed across these strata.
Finally, to minimize variability due to competing water systems per city, we restricted our analyses to cities that reported water service from a single system, in which exposure to lead would likely be more uniform. To minimize the effects of smaller water systems, we restricted modeling to systems serving more than 1000 people. We also restricted to cities with lead levels below the current EPA actionable lead level (<0.015 mg/L).
Analyses were performed using JMP Pro and SAS (Cary, NC).
Results
Of 597,968 patients across the United States who began dialysis between 2005 and 2017, 86% lived in cities with detectable 90th percentile lead levels, and 2% in cities with community water lead above the EPA’s actionable level (0.015 mg/L). Patient characteristics were generally similar across lead categories, except for less diabetes and more congestive heart failure among patients residing in cities with higher levels (Table 1).
Table 1.
Patient and city water system characteristics according to levels of lead in the community water in a patient’s city of primary residence
| Characteristic | Lead in Community Water (mg/L) | ||||
|---|---|---|---|---|---|
| <0.001 | 0.001 to <0.00375 | 0.00375 to <0.0075 | 0.0075 to <0.015 | ≥0.015 | |
| Number of patients | 80,851 | 252,762 | 173,379 | 78,307 | 12,669 |
| Number of cities | 3050 | 6806 | 5109 | 2085 | 702 |
| Number of dialysis facilities | 4420 | 6247 | 5555 | 4063 | 1749 |
| Patient characteristics pre-ESKD | |||||
| Age, yr | 62.5 (15.4) | 62.8 (15.6) | 62.8 (15.7) | 63.0 (15.8) | 63.3 (15.7) |
| Female, n | 43 | 43 | 43 | 43 | 42 |
| Race, n | |||||
| White | 64 | 67 | 68 | 63 | 70 |
| Black | 26 | 27 | 26 | 31 | 27 |
| Asian | 6 | 3 | 3 | 4 | 2 |
| Other/Unknown | 3 | 2 | 2 | 4 | 1 |
| Comorbidities, n | |||||
| Diabetes | 57 | 55 | 54 | 54 | 53 |
| Hypertension | 88 | 87 | 87 | 86 | 87 |
| Congestive heart failure | 29 | 30 | 32 | 32 | 34 |
| Cancer | 7 | 8 | 8 | 8 | 8 |
| Tobacco use | 7 | 7 | 6 | 6 | 7 |
| Body mass index | 29.6 (8.1) | 29.4 (8.0) | 28.9 (7.9) | 28.9 (7.9) | 29.2 (8.1) |
| eGFR, ml/min per m2 | 10.2 (4.6) | 10.4 (4.7) | 10.4 (4.8) | 10.3 (4.8) | 10.0 (4.6) |
| Full- or part-time employment, n | 36 | 35 | 35 | 37 | 34 |
| Uninsured, n | 6 | 6 | 6 | 5 | 6 |
| Air small particulate concentration, µg/m3, per county | 12.1 (1.4) | 12.0 (1.5) | 11.9 (1.4) | 11.9 (1.3) | 11.9 (1.4) |
| City water system characteristics | |||||
| Number of water systems per city | 4.4 (9.8) | 6.9 (15.7) | 4.5 (8.9) | 3.9 (8.7) | 3.4 (6.7) |
| Number of lead samples reported | 23.2 (38.6) | 45.8 (98.1) | 32.5 (50.8) | 31.9 (39.8) | 28.4 (25.9) |
| Average persons served (1000) | 78.6 (161.0) | 122.0 (241.8) | 186.5 (458.6) | 895.1 (1483.9) | 86 (170.5) |
Means (standard deviations) for continuous variables and column percentages for categorical variables provided.
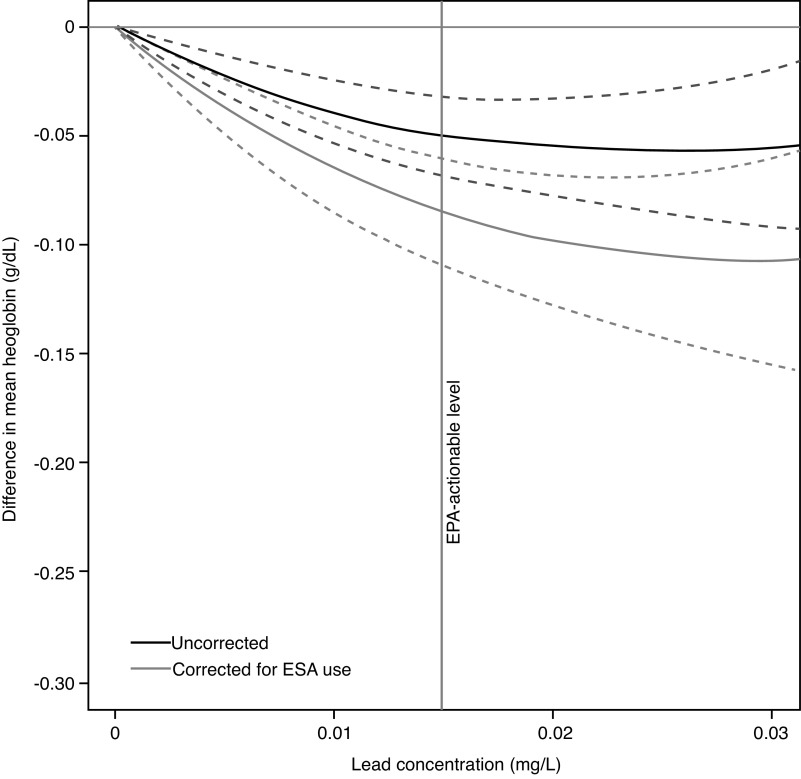
The average pre-ESKD hemoglobin was 9.77 (1.64) g/dl, and when corrected for pre-ESKD ESA use, 9.23 (1.85) g/dl. In adjusted analyses (main models, Table 2, Figure 1; progressive models Supplemental Table 1), community water lead levels were associated with lower hemoglobin concentrations, with a 0.02 g/dl (95% confidence interval [95% CI], 0.01 to 0.02) lower hemoglobin concentration for each 0.01 mg/L increment in community water lead. The corresponding estimate for ESA-corrected hemoglobin concentration was 0.03 g/dl (95% CI, 0.02 to 0.04) lower.
Table 2.
Adjusted difference (95% CI) in hemoglobin concentrations and prevalence of ESA use according to the 90th percentile community water lead concentration in a patient’s city of primary residence
| Outcome | Categories of Increasing Lead Concentrations (mg/L) | Per 0.01 mg/L | |||||
|---|---|---|---|---|---|---|---|
| <0.001 | 0.001 to <0.00375 | 0.00375 to <0.0075 | 0.0075 to <0.015 | ≥0.015 | |||
| Adjusted difference in pre-ESKD hemoglobin concentration | |||||||
| Hemoglobin (597,968) | Ref. | −0.02 g/dl | −0.007 g/dl | −0.05 g/dl | −0.04 g/dl | −0.02 g/dl | |
| (−0.03 to 0.00) | (−0.02 to 0.01) | (−0.07 to −0.03) | (−0.07 to −0.01) | (−0.02 to −0.01) | |||
| Hemoglobin corrected for pre-ESKD ESA use (449,205) | Ref. | −0.002 g/dl | −0.01 g/dl | −0.05 g/dl | −0.09 g/dl | −0.03 g/dl | |
| (−0.02 to 0.02) | (−0.03 to 0.01) | (−0.08 to −0.03) | (−0.13 to −0.05) | (−0.04 to −0.02) | |||
| Adjusted difference in hemoglobin concentrations during the first month of dialysis | |||||||
| Hemoglobin (208,912) | Ref. | −0.04 | −0.06 g/dl | −0.12 g/dl | −0.14 g/dl | −0.05 g/dl | |
| (−0.06 to −0.02) | (−0.08 to −0.04) | (−0.15 to −0.09) | (−0.19 to −0.09) | (−0.06 to −0.04) | |||
| Hemoglobin corrected for ESA use during first month of dialysis (203,006) | Ref. | −0.05 | −0.04 g/dl | −0.13 g/dl | −0.14 g/dl | −0.05 g/dl | |
| (−0.08 to −0.03) | (−0.07 to −0.02) | (−0.17 to −0.10) | (−0.21 to −0.08) | (−0.06 to −0.03) | |||
| Adjusted difference in ESA use | |||||||
| Pre-ESKD ESA use (449,205) | Ref. | −0.3% | 0.2% | 0.04% | 1.9% | 0.4% | |
| (−0.7 to 0.4) | (−0.2 to 0.6) | (−0.43 to 0.50) | (1.0 to 2.8) | (0.2 to 0.6) | |||
| ESA use during first month of dialysis (203,006) | Ref. | 0.3% | −0.5% | 1.2% | 0.8% | 0.3% | |
| (−0.3 to 0.9) | (−1.2 to 0.3) | (0.3 to 2.1) | (−0.9 to 2.5) | (−0.1 to 0.6) | |||
To account for the effect of ESA therapy, corrected hemoglobins were derived by subtracting 2 g/dl from hemoglobin concentrations among pre-ESKD ESA users and among those receiving ESA during the first month of dialysis. Adjusted for age, sex, race, history of diabetes, congestive heart failure, hypertension, cancer, tobacco use, body mass index, estimated glomerular function, insurance status, employment, year of dialysis initiation, and county air pollution.
Figure 1.
Cubic polynomials were fitted to illustrate the adjusted difference in pre-ESKD hemoglobin concentration, according to the 90th percentile community water lead concentration in the patient’s city of primary residence. To account for the effect of ESA treatment, corrected hemoglobin was derived by subtracting 2 g/dl from observed hemoglobin concentration among pre-ESKD ESA users. Adjusted for age, sex, race, history of diabetes, congestive heart failure, hypertension, cancer, tobacco use, body mass index, estimated glomerular function, insurance status, employment, year of dialysis initiation, and county air pollution. Dashed lines reflect 95% CI. The figure excludes patients (n=3187) in cities with a reported lead concentration ≥0.03 mg/L.
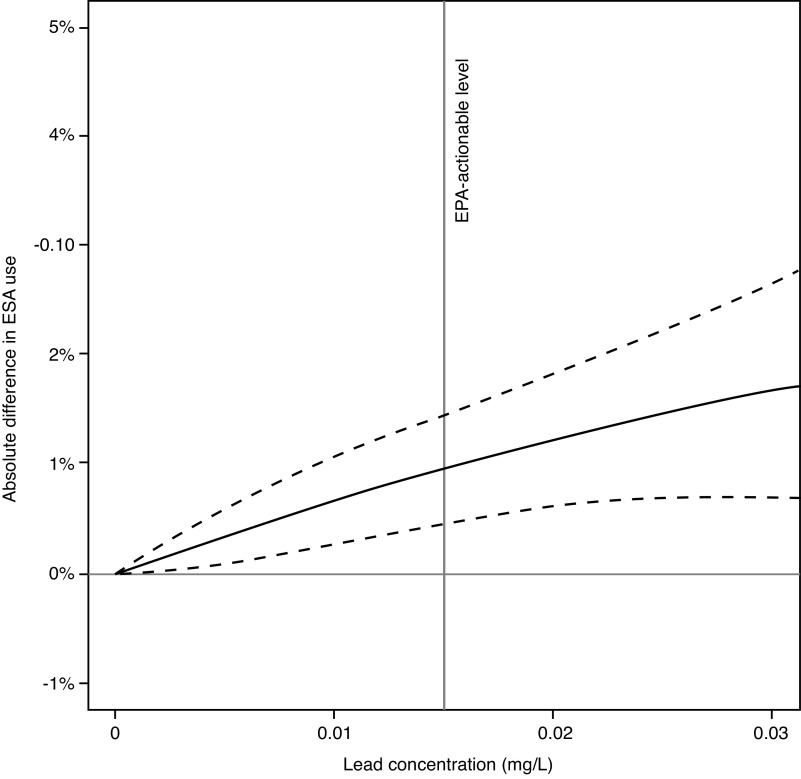
ESA therapy was administered to 29% patients before dialysis initiation; the corresponding percentages across increasing categories of lead concentrations were 27%, 28%, 30%, 31%, and 35%. The adjusted use of ESA therapy increased with higher community water lead concentrations; a 0.01 mg/L higher lead concentration was associated with a 0.4% (95% CI, 0.2% to 0.6%) increment in absolute prevalence of pre-ESKD ESA therapy (Table 2, Figure 2).
Figure 2.
Cubic polynomials were fitted to illustrate the adjusted difference in ESA use before dialysis initiation, according to the 90th percentile community water lead concentration in a patient’s city of primary residence. Adjusted for age, sex, race, history of diabetes, congestive heart failure, hypertension, cancer, tobacco use, body mass index, estimated glomerular function, insurance status, employment, year of dialysis initiation, and county air pollution. Dashed lines reflect 95% CI. The figure excludes patients in cities with lead concentrations ≥0.03 mg/L.
Data from the first month of chronic dialysis therapy was available for 208,912 patients. Higher levels of community water lead were similarly associated with lower hemoglobin and higher prevalence of ESA utilization (Table 2, Supplemental Figures 2 and 3).
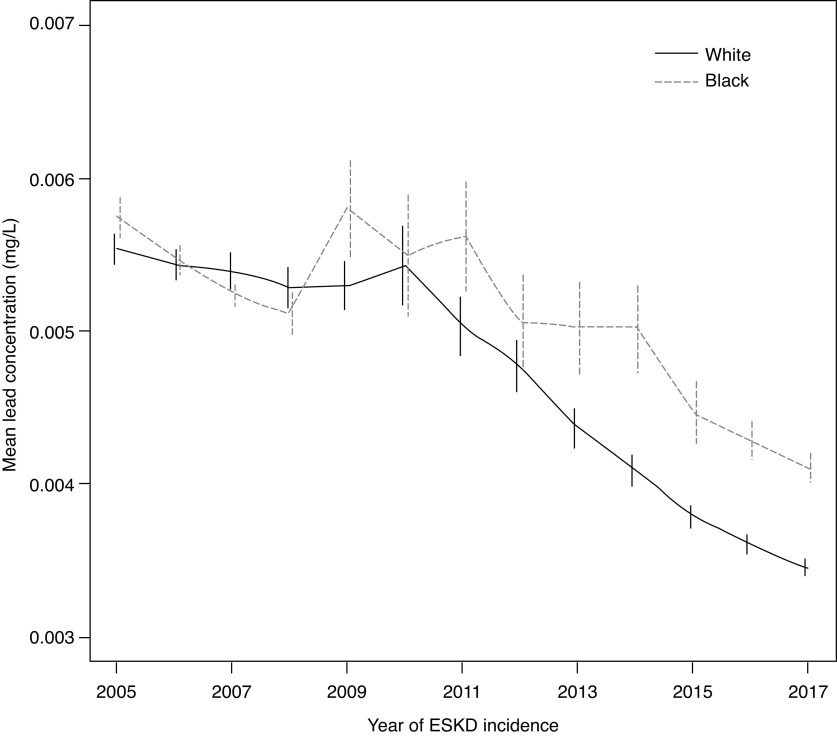
Community water lead levels were highest among Black patients and those without health insurance (Table 3). There were significant differences in the temporal trends of water lead levels for Black compared with White patients (P<0.001) (Figure 3). Across the period of study, community water levels decreased by 0.0002 mg/L per year among White patients, compared with 0.0001 mg/L per year among Black patients. The adjusted change in hemoglobin per 0.01 mg/L higher 90th percentile community water was higher among those lacking health insurance, although not significantly different, and across counties with an increasing prevalence of pediatric lead toxicity.
Table 3.
The 90th percentile community water lead levels, and the adjusted change in hemoglobin per 0.01 higher mg/L lead, according to race, socioeconomic factors, and prevalence of documented patients with of lead toxicity
| Characteristics | 90th Percentile Community Water Lead Concentrations (mg/L) | Adjusted Change in Hemoglobin per 0.01 mg/L Higher 90th Percentile Community Water Leada | ||
|---|---|---|---|---|
| Value | P value testing strata differencesb | Estimate (95% CI) | P value testing strata differencesc | |
| Race | ||||
| White | 0.0047 | <0.001 | −0.02 (−0.03 to −0.01) | 0.14 |
| Black | 0.0051 | −0.01 (−0.03 to 0.00) | ||
| Asian | 0.0042 | −0.03 (−0.07 to 0.02) | ||
| Insurance status | ||||
| Insured | 0.0048 | 0.05 | −0.02 (−0.02 to −0.01) | 0.16 |
| Uninsured | 0.0049 | −0.04 (−0.06 to −0.01) | ||
| Employment | ||||
| Employed | 0.0048 | 0.63 | −0.02 (−0.03 to −0.01) | 0.96 |
| Unemployed | 0.0048 | −0.02 (−0.03 to −0.01) | ||
| County level estimates of pediatric lead toxicity (percentiles) | ||||
| <75th | 0.0039 | <0.001 | −0.003 (−0.02 to 0.01) | 0.007 |
| ≥75th <90th | 0.0051 | −0.005 (−0.02 to 0.01) | ||
| ≥90th | 0.0039 | −0.12 (−0.19 to −0.05) | ||
aAdjusted for age, sex, race, history of diabetes, congestive heart failure, hypertension, cancer, tobacco use, body mass index, estimated glomerular function, insurance status, employment, year of dialysis initiation and county air pollution.
bP values represent group differences as tested by a one-way analysis of variance.
cP values represent group differences as tested by multiplicative interaction terms between 0.01 mg/L lead and determinant.
Figure 3.
Temporal trends in the mean (95% CI) 90th percentile community water lead concentrations for Black and White patients with ESKD were significantly different (P<0.001). The adjusted per calendar year change in lead levels was 0.0002 mg/L lower per year among White patients, compared with 0.0001 mg/L lower per year among Black patients.
When restricted to 5993 cities served by a single community water system (n=260,490 patients), a 0.01 mg/L increment in lead level was associated with 0.02 g/dl (95% CI, 0.01 to 0.03) lower pre-ESKD hemoglobin concentration, but was not associated with pre-ESKD ESA use (difference, 0.1%; 95% CI, -0.2 to 0.3). When restricted to 9077 larger community water systems (serving more than 1000 individuals) in 7532 cities (n=570,916 patients), a 0.01 mg/L increment in lead was associated with 0.03 g/dl (95% CI, 0.02 to 0.03) lower pre-ESKD hemoglobin concentration and 0.5% (95% CI, 0.2 to 0.7) higher prevalence of pre-ESKD ESA use. When restricted to 585,299 individuals living in cities with lead levels below the EPA actionable level (0.015 mg/L), a 0.01 mg/L increment in lead level remained associated with 0.05 g/dl (95% CI, 0.03 to 0.06) lower pre-ESKD hemoglobin concentration and 0.4% (95% CI, 0.0 to 0.8) higher prevalence of pre-ESKD ESA use.
Discussion
Among patients in the United States who initiated dialysis between 2005 and 2017, those living in cities with higher levels of lead in the community water had significantly lower hemoglobin concentrations and more ESA use. These associations were observed across the range of lead levels commonly found in community water across the United States and well below the current actionable level set by the EPA.
Our findings suggest that for patients with kidney disease, there is no safe amount of lead in drinking water. Although water has generally been considered a minor cause of lead toxicity,37–40 increased absorption and decreased excretion in those with kidney disease confer an exaggerated susceptibility.41,42 Given the prolonged t1/2 and extensive soft tissue and skeletal deposition of ingested lead,43–46 chronic exposure results in progressive accumulation with consequences that may manifest after many years.47,48 The downstream effects of lower hemoglobin concentrations, including fatigue, need for blood transfusions, and cardiovascular disease, and the known adverse risks and financial burden associated with chronic ESA use,49 underscore the scope of hazard attributable to drinking contaminated water. Furthermore, because lead has a range of detrimental effects,50 whether levels commonly found in water might associate with other diseases highly prevalent in the CKD population, including CKD of uncertain etiology,51 depression,52 and dementia53 will require further research. Although the toxic effects of other metals have been described in CKD,54–57 few studies have evaluated the epidemiologic association of heavy metals in drinking water and chronic disease. Because large proportions of the United States population are exposed to lead in the drinking water, the public health consequences of our findings are enormously important.
Our findings raise broader concerns about the aging water system infrastructure in the United States. The full of extent of lead contamination is unknown, in part due to large numbers of lead lines that remain in service and older household plumbing. In addition, because federal regulations require water systems to nonrandomly sample a small proportion of households, and report only the 90th percentile of those values, there is no nationally representative measure of water quality at the household level. Accordingly, without accurate estimation of individual levels of exposure, further research is greatly hampered.
The hazard of failing to understand the full scope of lead contamination of water systems across the United States is particularly poignant for minorities. Black patients have increased susceptibility to lead exposure due to higher rates of kidney disease and lower access to equitable health care,58–60 and, simultaneously, greater vulnerability, as evidenced by the higher levels of community water lead levels among Black than White patients with ESKD, with significantly less temporal improvement over the last decade. Circulating lead levels and rates of lead toxicity are known to be higher among Black patients,61–63 and the role of contaminated water to this disparity will require further study. However, as suggested by investigative and academic studies,64–67 the current system of water surveillance may not only fail to accurately detect lead contamination, but also systematically underestimate exposure in the most vulnerable communities. That the effect of lead exposure on hemoglobin was significantly greater in counties with greater proportions of pediatric lead toxicity suggests the water system level lead measures may underestimate the true exposure at the individual level. Although other sources of lead exposure might contribute to the strata-specific differences, lead paint ingestion is unlikely in a largely adult ESKD population.
In addition to the absence of individual measures of drinking water lead, our analysis has other limitations. We could not account for how much water an individual drank, the use of bottled or filtered water, and the length of primary residence. In addition, averaging the level of exposure for all residents in cities with multiple water systems likely introduces misclassification, although estimates were consistent in cities with only one water system and when restricted to larger water systems. Although our models adjusted for a wide range of exposures, additional determinants of hemoglobin concentrations, including exposure to other sources of lead, could reflect unaccounted confounding. Levels of lead in the blood would help clarify the risk of lead toxicity associated with water exposure, but have not been widely measured in the ESKD population. In addition, variability in water system reporting of aberrant lead values likely leads to exposure misclassification. Finally, the design of our study makes it impossible to determine the specific mechanisms by which lead levels in community water may lead to anemia. Although it may reflect direct hematologic toxicity from lead, it is noteworthy that lead also impairs kidney function, which can then further exacerbate anemia. Regardless of mechanism, however, our results do suggest even low levels of lead can have hematologic consequences in vulnerable patients.
Elevations of lead commonly found in United States community water are associated with lower hemoglobin concentrations and higher use of ESA among patients with advanced kidney disease. Whether similar levels of lead exposure are associated with other lead-related diseases or among patients with less-advanced kidney disease warrants study.
Disclosures
E.D. Weinhandl reports having consultancy agreements with Fresenius Medical Care North America; reports being a scientific advisor or member of the Advisory Board of Home Dialyzors United, Board of Directors member of Medical Education Institute; and reports having other interests/relationships as member of the Scientific Methods Panel for the National Quality Forum. J. Danziger reports having other interests/relationships as former Medical Director of NxStage Boston South Dialysis Unit, and ongoing medical legal consulting. K.J. Mukamal reports having other interests/relationships with the US Highbush Blueberry Council and Wolters Kluwer.
Funding
None.
Acknowledgments
Dr. J. Danziger designed the study; E.D. Weinhandl was responsible for statistical analyses; and all authors drafted and revised the manuscript and approved the final version.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Racial inequalities in drinking water lead exposure: A wake-up call to protect patients with end stage kidney disease,” on pages 2419–2421.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2020091281/-/DCSupplemental.
Supplemental Table 1. Effect of adjustment for patient characteristics, socioeconomic determinants, and environmental air quality, on the association of a 0.01 mg/L higher 90th percentile lead level on hemoglobin concentrations and erythropoietin stimulating agent use.
Supplemental Figure 1. Flow chart outlining the merge of Safe Drinking Water Information System and United States Renal Data System to create a complete dataset of 597,968 unique patients with ESKD.
Supplemental Figure 2. Adjusted difference in hemoglobin concentration during the first month of dialysis, according to the 90th percentile drinking water lead concentration in a patient’s city of primary residence. To account for the effect of ESA treatment, corrected hemoglobin was derived by subtracting 2 g/dl from observed hemoglobin concentration among ESA users during the first month of dialysis. Adjusted for age, sex, race, history of diabetes, congestive heart failure, hypertension, cancer, tobacco use, body mass index, estimated glomerular function, insurance status, employment, year of dialysis initiation, and county air pollution. Dashed lines reflect 95% CI. The figure excludes patients in cities with reported lead concentration ≥0.03 mg/L.
Supplemental Figure 3. Adjusted difference in ESA use during the first month of dialysis, according to the 90th percentile drinking water lead concentration in a patient’s city of primary residence. Adjusted for age, sex, race, history of diabetes, congestive heart failure, hypertension, cancer, tobacco use, body mass index, estimated glomerular function, insurance status, employment, year of dialysis initiation, and county air pollution. The figure excludes patients in cities with lead concentrations ≥0.03 mg/L.
References
- 1.Alarcon WA; State Adult Blood Lead Epidemiology and Surveillance (ABLES) Program Investigators: Elevated blood lead levels among employed adults – United States, 1994–2013. MMWR Morb Mortal Wkly Rep 63: 59–65, 2016 [DOI] [PubMed] [Google Scholar]
- 2.US Environmental Protection Agency: Protect your family from sources of lead. Available at: https://www.epa.gov/lead/protect-your-family-sources-lead. Accessed November 1, 2021
- 3.Centers for Disease Control and Prevention: Lead in drinking water. Available at: www.cdc.gov/nceh/lead/tips/water. Accessed January 11, 2021
- 4.US Environmental Protection Agency: Understanding the lead and copper rule. Available at: https://www.epa.gov/sites/production/files/2019-10/documents/lcr101_factsheet_10.9.19.final_.2.pdf. Accessed January 5, 2020
- 5.Lin-Tan DT, Lin JL, Yen TH, Chen KH, Huang YL: Long-term outcome of repeated lead chelation therapy in progressive non-diabetic chronic kidney diseases. Nephrol Dial Transplant 22: 2924–2931, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Yen TH, Lin-Tan DT, Lin JL: Chronic renal failure induced by lead. Kidney Int 79: 688, 2011 [DOI] [PubMed] [Google Scholar]
- 7.Bennett WM: Lead nephropathy. Kidney Int 28: 212–220, 1985 [DOI] [PubMed] [Google Scholar]
- 8.Batuman V, Wedeen RP: Impairment of renal function with increasing blood lead concentrations. N Engl J Med 327: 1394–1395, 1992 [PubMed] [Google Scholar]
- 9.Kim R, Rotnitsky A, Sparrow D, Weiss S, Wager C, Hu H: A longitudinal study of low-level lead exposure and impairment of renal function: The Normative Aging Study. JAMA 275: 1177–1181, 1996 [PubMed] [Google Scholar]
- 10.Staessen JA, Lauwerys RR, Buchet J-P, Bulpitt CJ, Rondia D, Vanrenterghem Y, et al. ; The Cadmibel Study Group: Impairment of renal function with increasing blood lead concentrations in the general population. N Engl J Med 327: 151–156, 1992 [DOI] [PubMed] [Google Scholar]
- 11.Blake KCH, Mann M: Effect of calcium and phosphorus on the gastrointestinal absorption of 203Pb in man. Environ Res 30: 188–194, 1983 [DOI] [PubMed] [Google Scholar]
- 12.Barton JC, Conrad ME, Nuby S, Harrison L: Effects of iron on the absorption and retention of lead. J Lab Clin Med 92: 536–547, 1978 [PubMed] [Google Scholar]
- 13.Barton JC, Conrad ME, Harrison L, Nuby S: Effects of calcium on the absorption and retention of lead. J Lab Clin Med 91: 366–376, 1978 [PubMed] [Google Scholar]
- 14.Muntner P, He J, Vupputuri S, Coresh J, Batuman V: Blood lead and chronic kidney disease in the general United States population: results from NHANES III. Kidney Int 63: 1044–1050, 2003 [DOI] [PubMed] [Google Scholar]
- 15.Lee SH, Huang JW, Hung KY, Leu LJ, Kan YT, Yang CS, et al. : Trace metals’ abnormalities in hemodialysis patients: Relationship with medications. Artif Organs 24: 841–844, 2000 [DOI] [PubMed] [Google Scholar]
- 16.Palaneeswari MS, Abraham Sam Rajan PM, Santhi S, Jothimalar: Blood lead in end-stage renal disease (ESRD) patients who were on maintenance haemodialysis. J Clin Diagnostic Res 6: 1633–1365, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sargent JD, Meyers A, Weitzman M, Bellinger D, Sloman J, Leviton A, et al. : Environmental exposure to lead and cognitive deficits in children. N Engl J Med 320: 595–596, 1989 [DOI] [PubMed] [Google Scholar]
- 18.Tong S, Baghurst P, McMichael A, Sawyer M, Mudge J: Lifetime exposure to environmental lead and children’s intelligence at 11–13 years: The Port Pirie cohort study. BMJ 312: 1569–1575, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gonick HC: Lead, renal disease and hypertension. Am J Kidney Dis 40: 202–204, 2002 [DOI] [PubMed] [Google Scholar]
- 20.Heaney RP: Blood lead levels and hypertension. JAMA 290: 460–461 2003 [DOI] [PubMed] [Google Scholar]
- 21.Ding Y, Vaziri ND, Gonick HC: Lead-induced hypertension. II. Response to sequential infusions of L-arginine, superoxide dismutase, and nitroprusside. Environ Res 76: 107–113, 1998 [DOI] [PubMed] [Google Scholar]
- 22.Dundar B, Oktem F, Arslan MK, Delibas N, Baykal B, Arslan C, et al.: The effect of long-term low-dose lead exposure on thyroid function in adolescents. Environ Res 101: 140-145, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Kahn LG, Liu X, Rajovic B, Popovac D, Oberfield S, Graziano JH, et al. : Blood lead concentration and thyroid function during pregnancy: Results from the Yugoslavia Prospective Study of Environmental Lead Exposure. Environ Health Perspect 122: 1134–1140, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goyer RA, Rhyne BC: Pathological effects of lead. Int Rev Exp Pathol 12: 1–77, 1973 [PubMed] [Google Scholar]
- 25.Bradman A, Eskenazi B, Sutton P, Athanasoulis M, Goldman LR: Iron deficiency associated with higher blood lead in children living in contaminated environments. Environ Health Perspect 109: 1079–1084, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Turgut S, Polat A, Inan M, Turgut G, Emmungil G, Bican M, et al. : Interaction between anemia and blood levels of iron, zinc, copper, cadmium and lead in children. Indian J Pediatr 74: 827–830, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Gellert GA, Wagner GA, Maxwell RM, Moore D, Foster L: Lead poisoning among low-income children in Orange County, California: A need for regionally differentiated policy. JAMA 270: 69–71, 1993 [PubMed] [Google Scholar]
- 28.Adams WG, Geva J, Coffman J, Palfrey S, Bauchner H: Anemia and elevated lead levels in underimmunized inner-city children. Pediatrics 101: E6, 1998 [DOI] [PubMed] [Google Scholar]
- 29.Recasens V, Montañés A, Bustamante E: Lead poisoning as final diagnosis in a study of normocytic anemia. Int J Hematol 109: 135–136, 2019 [DOI] [PubMed] [Google Scholar]
- 30.Hsieh NH, Chung SH, Chen SC, Chen WY, Cheng YH, Lin YJ, et al. : Anemia risk in relation to lead exposure in lead-related manufacturing. BMC Public Health 17: 389, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bashir R, Khan DA, Saleem M, Zaman KU, Malik IA: Blood lead levels and anemia in lead exposed workers. J Pak Med Assoc 45: 64–66, 1995 [PubMed] [Google Scholar]
- 32.US Environmental Protection Agency: Safe Drinking Water Information System (SDWIS) Federal Reporting Services. Available at: https://www.epa.gov/ground-water-and-drinking-water/safe-drinking-water-information-system-sdwis-federal-reporting. Accessed October 1, 2020
- 33.Electronic Code of Federal Regulations: Part 141: National Primary Drinking Water Regulations; 141.80. Available at : https://www.ecfr.gov. Accessed December 1, 2020
- 34.Tobin MD, Sheehan NA, Scurrah KJ, Burton PR: Adjusting for treatment effects in studies of quantitative traits: antihypertensive therapy and systolic blood pressure. Stat Med 24: 2911–2935, 2005 [DOI] [PubMed] [Google Scholar]
- 35.Jones M, Ibels L, Schenkel B, Zagari M: Impact of epoetin alfa on clinical end points in patients with chronic renal failure: A meta-analysis. Kidney Int 65: 757–767, 2004 [DOI] [PubMed] [Google Scholar]
- 36.Centers for Disease Control and Prevention: Wide-ranging Online Data for Epidemiologic Research, Center for Disease Control and Research. Available at: https://wonder.cdc.gov/nasa-pm.html. Accessed December 10, 2020
- 37.Dong Z, Hu J: Development of lead source-specific exposure standards based on aggregate exposure assessment: Bayesian inversion from biomonitoring information to multipathway exposure. Environ Sci Technol 46: 1144–1152, 2012 [DOI] [PubMed] [Google Scholar]
- 38.Moore MR, Meredith PA, Campbell BC, Goldberg A, Pocock SJ: Contribution of lead in drinking water to blood-lead. Lancet 2: 661–662, 1977 [DOI] [PubMed] [Google Scholar]
- 39.Sherlock JC, Ashby D, Delves HT, Forbes GI, Moore MR, Patterson WJ, et al. : Reduction in exposure to lead from drinking water and its effect on blood lead concentrations. Hum Toxicol 3: 383–392, 1984 [DOI] [PubMed] [Google Scholar]
- 40.Brown MJ, Margolis S.. Lead in drinking water and human blood lead levels in the United States. Morbid Mortal Week Rep 61 (Suppl): 1–9, 2012 [PubMed] [Google Scholar]
- 41.Rabinowitz MB, Wetherill GW, Kopple JD: Lead metabolism in the normal human: Stable isotope studies. Science 182: 725–727, 1973. [DOI] [PubMed] [Google Scholar]
- 42.Rabinowitz MB, Wetherill GW, Kopple JD: Kinetic analysis of lead metabolism in healthy humans. J Clin Invest 58: 260–270, 1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Heard MJ, Chamberlain AC: Uptake of Pb by human skeleton and comparative metabolism of Pb and alkaline earth elements. Health Phys 47: 857–865, 1984 [DOI] [PubMed] [Google Scholar]
- 44.Ziegler EE, Edwards BB, Jensen RL, Mahaffey KR, Fomon SJ: Absorption and retention of lead by infants. Pediatr Res 12: 29–34, 1978 [DOI] [PubMed] [Google Scholar]
- 45.Manton WI, Angle CR, Stanek KL, Reese YR, Kuehnemann TJ: Acquisition and retention of lead by young children. Environ Res 82: 60–80, 2000 [DOI] [PubMed] [Google Scholar]
- 46.Shadick NA, Kim R, Weiss S, Liang MH, Sparrow D, Hu H: Effect of low level lead exposure on hyperuricemia and gout among middle aged and elderly men: The normative aging study. J Rheumatol 27: 1708–1712, 2000 [PubMed] [Google Scholar]
- 47.Reuben A, Schaefer JD, Moffitt TE, Broadbent J, Harrington H, Houts RM, et al. : Association of childhood lead exposure with adult personality traits and lifelong mental health. JAMA Psychiatry 76: 418–425, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Potula V, Serrano J, Sparrow D, Hu H: Relationship of lead in drinking water to bone lead levels twenty years later in Boston men: The Normative Aging Study. J Occup Environ Med 41: 349–355, 1999 [DOI] [PubMed] [Google Scholar]
- 49.Singh AK, Szczech L, Tang KL, Barnhart H, Sapp S, Wolfson M, et al. ; CHOIR Investigators : Correction of anemia with epoetin alfa in chronic kidney disease. N Engl J Med 355: 2085–2098, 2006 [DOI] [PubMed] [Google Scholar]
- 50.Mason LH, Harp JP, Han DY: Pb neurotoxicity: Neuropsychological effects of lead toxicity. BioMed Res Int 2014: 1-8, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lunyera J, Mohottige D, Von Isenburg M, Jeuland M, Patel UD, Stanifer JW: CKD of uncertain etiology: A systematic review. Clin J Am Soc Nephrol 11: 379–385, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bouchard MF, Bellinger DC, Weuve J, Matthews-Bellinger J, Gilman SE, Wright RO, et al. : Blood lead levels and major depressive disorder, panic disorder, and generalized anxiety disorder in US young adults. Arch Gen Psychiatry 66: 1313–1319, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bakulski KM, Rozek LS, Dolinoy DC, Paulson HL, Hu H: Alzheimer’s disease and environmental exposure to lead: The epidemiologic evidence and potential role of epigenetics. Curr Alzheimer Res 9: 563–573, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Alfrey AC, LeGendre GR, Kaehny WD: The dialysis encephalopathy syndrome. Possible aluminum intoxication. N Engl J Med 294: 184–188, 1976 [DOI] [PubMed] [Google Scholar]
- 55.Berend K, van der Voet G, Boer WH: Acute aluminum encephalopathy in a dialysis center caused by a cement mortar water distribution pipe. Kidney Int 59: 746–753, 2001 [DOI] [PubMed] [Google Scholar]
- 56.Kuo PH, Kanal E, Abu-Alfa AK, Cowper SE: Gadolinium-based MR contrast agents and nephrogenic systemic fibrosis. Radiology 242: 647–649, 2007 [DOI] [PubMed] [Google Scholar]
- 57.Zheng LY, Umans JG, Yeh F, Francesconi KA, Goessler W, Silbergeld EK, et al. : The association of urine arsenic with prevalent and incident chronic kidney disease: Evidence from the Strong Heart Study. Epidemiology 26: 601–612, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Danziger J, Ángel Armengol de la Hoz M, Celi LA, Cohen RA, Mukamal KJ: Use of do-not-resuscitate orders for critically ill patients with ESKD. J Am Soc Nephrol 31: 2393–2399, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Danziger J, Weinhandl E, Friedman D, Mukamal KJ: Racial and ethnic disparities in seasonal influenza vaccination among dialysis facilities in the United States. J Am Soc Nephrol 31: 2117–2121, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Danziger J, Ángel Armengol de la Hoz M, Li W, Komorowski M, Deliberato RO, Rush BNM, et al. : Temporal trends in critical care outcomes in U.S. minority-serving hospitals. Am J Respir Crit Care Med 201: 681–687, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Centers for Disease Control and Prevention: National Report on Human Exposure to Environmental Chemicals. 201. Available at : https://www.cdc.gov/exposurereport/pdf/FourthReport_UpdatedTables_Volume1_Jan2019-508.pdf. Accessed June 1, 2020
- 62.Fuortes LJ, Cowl CT, Reynolds SJ.. Ethnic and socioeconomic risk factors for lead toxicity. J Clean Tech Env Toxicol Occupat Med 6: 339–343, 1997 [Google Scholar]
- 63.Lin C, Kim R, Tsaih SW, Sparrow D, Hu H: Determinants of bone and blood lead levels among minorities living in the Boston area. Environ Health Perspect 112: 1147–1151, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Del Toral MA, Porter A, Schock MR: Detection and evaluation of elevated lead release from service lines: A field study. Environ Sci Technol 47: 9300–9307, 2013 [DOI] [PubMed] [Google Scholar]
- 65.Goovaerts P: Monitoring the aftermath of Flint drinking water contamination crisis: Another case of sampling bias? Sci Total Environ 590–591: 139–153, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Doré E, Deshommes E, Andrews RC, Nour S, Prévost M: Sampling in schools and large institutional buildings: Implications for regulations, exposure and management of lead and copper. Water Res 140: 110–122, 2018 [DOI] [PubMed] [Google Scholar]
- 67.Rosenthal L, Craft W: Buried lead: How the EPA has left Americans exposed to lead in drinking water. 2020. Available at: https://www.apmreports.org/story/2020/05/04/epa-lead-pipes-drinking-water. Accessed August 12, 2020