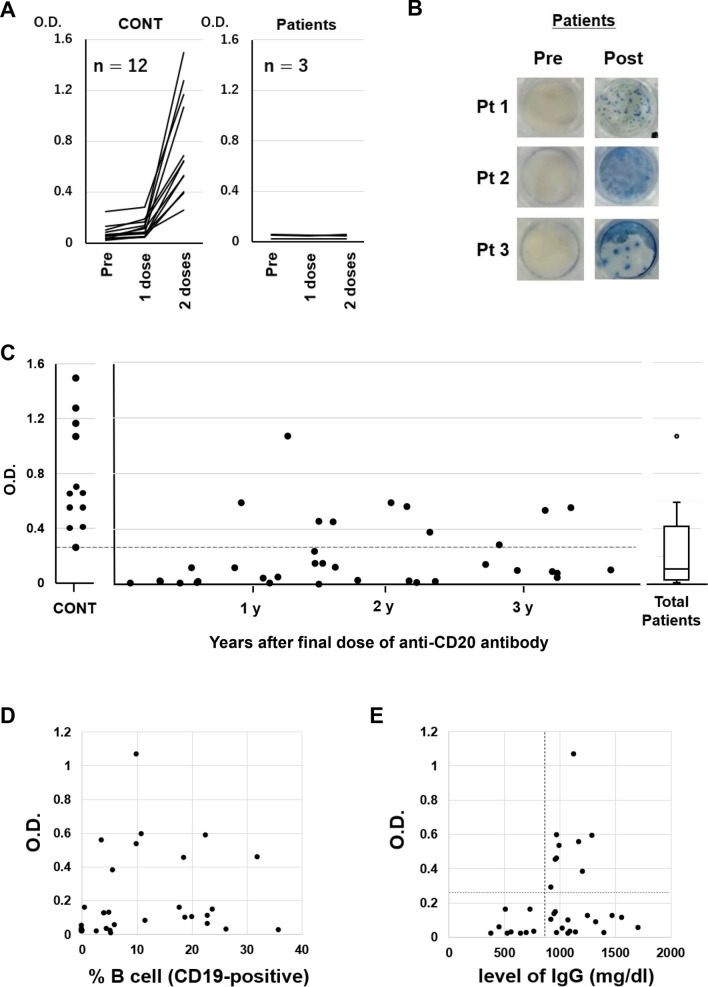
Fig. 1.
(A) Humoral quantitative anti-spike 1 (S1) antibody response at pre-vaccination (within 7 days prior to the first dose), 21 days (± 3 days) after the first dose and 14 days (± 7 days) after the second dose of BNT162b2 mRNA SARS-CoV-2 vaccine in healthy volunteers (n = 12) and diffuse large B-cell lymphoma patients treated with R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone) (n = 3). (B) Peripheral blood mononuclear cells obtained at pre-vaccination (within 7 days prior to the first dose) and 14 days (± 7 days) after the second dose of BNT162b2 from Patients 1–3 were stimulated overnight with SARS-CoV-2 spike peptide for ex vivo assessment by IFN-γ ELISPOT assay (human IFN-γ Single-Color Enzymatic ELISPOT Kit, Cellular Technology Limited, USA) according to the manufacturer's protocol. (C) S1 antibody titers were measured 14 days (± 7 days) after the second vaccination dose in 12 healthy donors and 36 patients who had received the final dose of anti-CD20 antibody 1–42 (median 17.5) months before vaccination. The horizontal dotted line indicates the lowest optical density (O.D.) value of S1 antibody titer in healthy donors. (D) Correlation between S1 antibody titer and percentage of CD19-positive B cells (normal range 6–23%). (E) Correlation between S1 antibody titer and total IgG level. The vertical dotted line indicates the lower normal limit of serum IgG. The horizontal dotted line indicates the lowest optical density (O.D.) value of S1 antibody titer in healthy donors