Fig. 4.
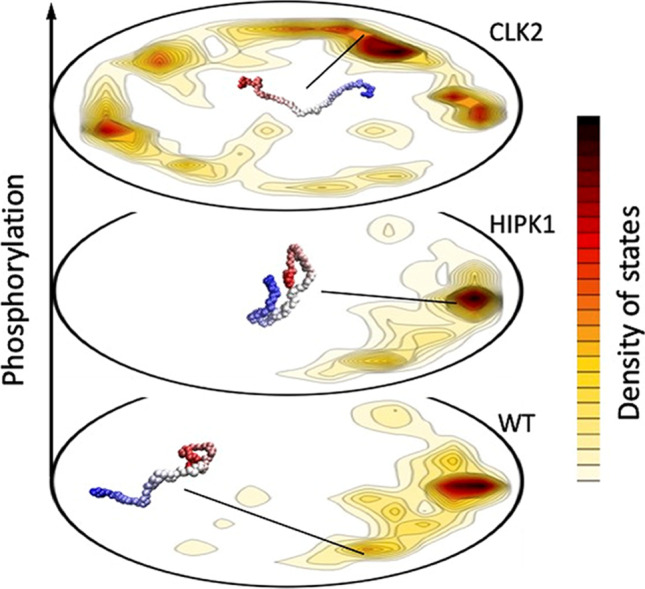
Employing the energy landscape visualization method (ELViM), different PAGE4 ensembles are represented in one single conformational phase space. The density of states, shown in the contour plots, varies according to the physical–chemical conditions, which in this case is the PAGE4 phosphorylation state. Each free energy valley can be characterized by specific conformations that entail particular binding affinities, typical of the promiscuous behavior of IDPs. For WT-PAGE4, through a fly-casting mechanism, the C-terminal region is extended, allowing the binding to its cognate partner. For the HIPK1-PAGE4, the lower free energy of the compact state decreases the affinity for c-Jun. Finally, the dominant extended conformations of CLK2-PAGE4 inhibit any binding affinity
