Fig. 4. Effects of NO gel treatment, 7 days following AVF creation in rats.
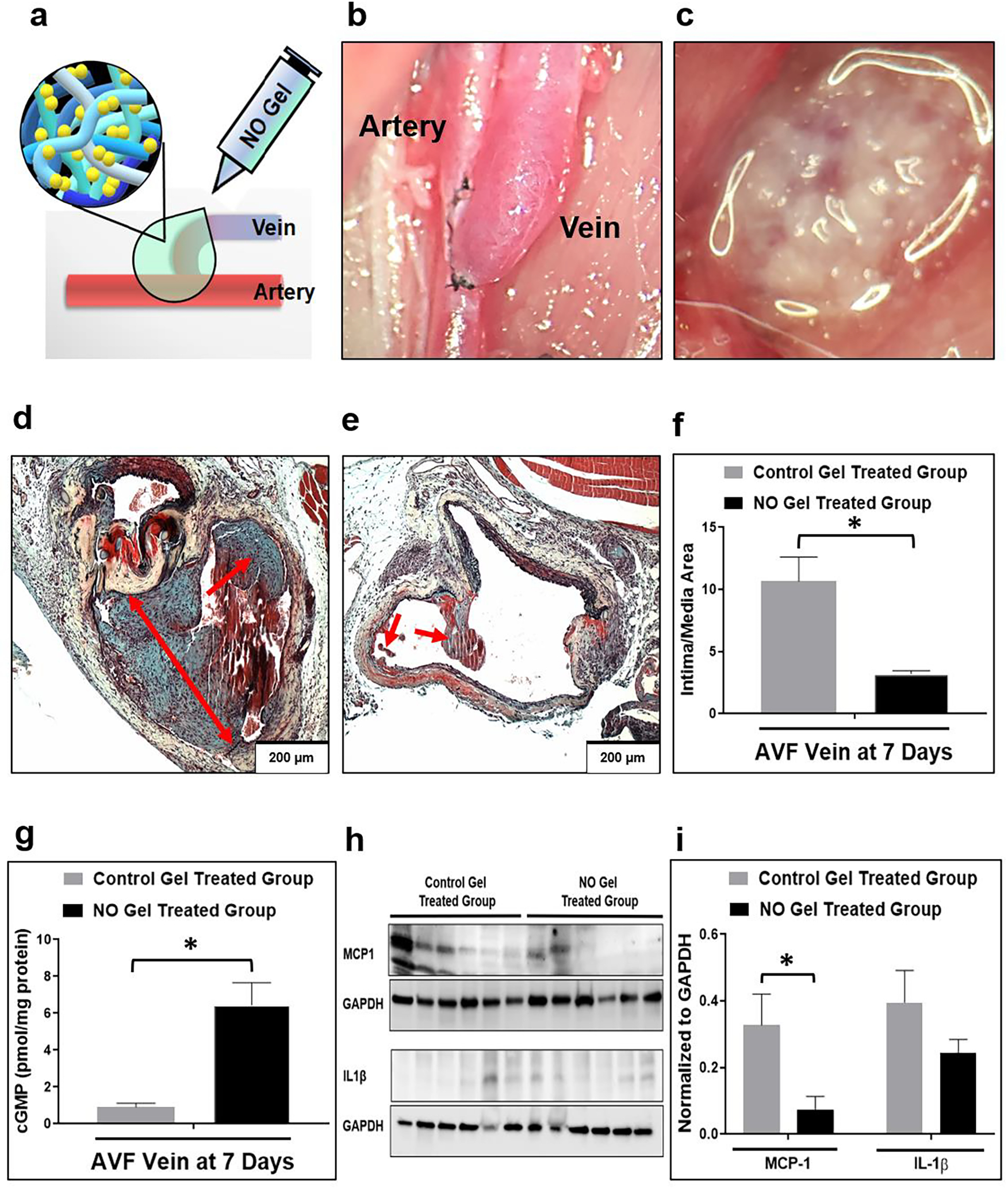
(a), (c) Application of NO releasing nanomatrix gel immediately after AVF creation. (b) Rat femoral (end) vein to artery (side) AVF model. (d) Representative histological images of venous intimal hyperplasia development following 7-day post-AVF creation with the control gel treated group (note the level of aggressive intimal hyperplasia) and (e) the NO releasing nanomatrix gel treated group (note the limited intimal hyperplasia formation). Arrows: Intimal hyperplasia development. (f) Morphometric analysis from AVF veins. Data are presented as the mean ± s.e.m. (n=4 for each group). *p<0.05. (g) cGMP concentration in AVF veins measured using ELISA. Data are presented as the mean ± s.e.m. (n=4 for control gel treated group and n=3 for NO gel treated group). *p<0.05. (h), (i) Representative Western blots and densitometric analysis of MCP-1 and IL-1β protein expression from AVF-veins. Equivalence of loading was assessed by immunoblotting for GAPDH. Data are presented as the mean ± s.e.m. (n=6 for each group). *p<0.05. An unpaired t-test was used to test for statistical differences between control gel treated and NO gel treated groups.
