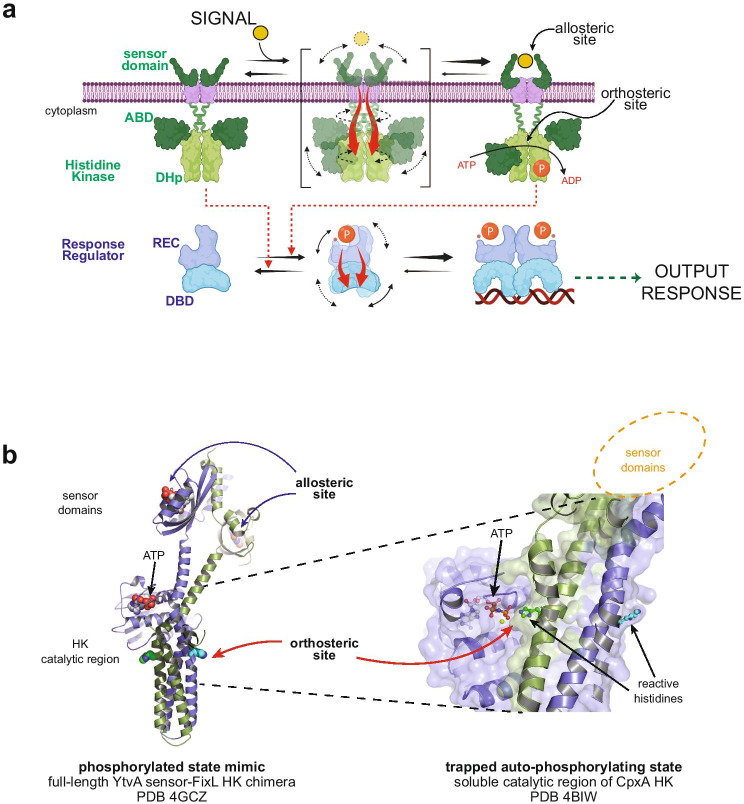
Fig. 2.
Allostery and protein plasticity in two-component systems. a Schematic drawing of two-component signal transduction systems in bacterial cells. The triggering signal is detected by the first protein component, a histidine kinase (HK). Stimuli are usually environmental (yellow sphere), yet intracellular cues can also be perceived by intracytoplasmic sensor domains, where the allosteric site is located. Conformational rearrangements are coupled to the orthosteric site, in this case located at the HK catalytic reaction center. Allosteric coupling pathways are depicted as red arrows (also for panels (a) and (b)). The conformational transition goes from a kinase-off/phosphatase-on state (upper, left) to kinase-on/phosphatase-off state (upper, right) that can autophosphorylate using ATP. A coiled-coil structure or S-helix serves as a transmission gear, highlighted in the transition state of the HK (upper, mid panel), affecting the catalytic center on the central “dimerization and histidine phosphotransfer” domain (DHp) and the mobility of the ATP-binding domains (ABDs). The downstream output response is executed by a distinct second protein component, the response regulator (RR). The non-covalent link is guaranteed by phosphoryl-transfer from the P ~ His on the HK, to the reactive Asp on the RR’s receiver (REC) domain. This phosphorylation allosterically shifts the equilibrium from the RR’s “inactive” state (lower, left) to a phosphorylated “activated” state (lower, right). The allosteric coupling to favor homo-dimerization and output response domain (in this case DNA-binding DBD) activation is highlighted in the lower, central panel. HK activities on the RR are depicted as red dotted arrows. Created with BioRender.com, b cartoon representations of histidine-kinases highlighting the spatial separation between the sensory allosteric sites and the orthosteric site engaged in catalysis. To the left, a functional full-length HK shows the overall location of key sites, with the right panel closing up on the orthosteric site trapped while performing auto-phosphorylation catalysis. Both panels correspond to crystal structures of different HKs as indicated, both belonging to the same family (HisKA). Signal-dependent changes at the allosteric site provoke reorganizations of the orthosteric site, ultimately regulating HK-catalyzed auto-phosphorylation, RR phosphoryl transfer, and P ~ RR dephosphorylation activities