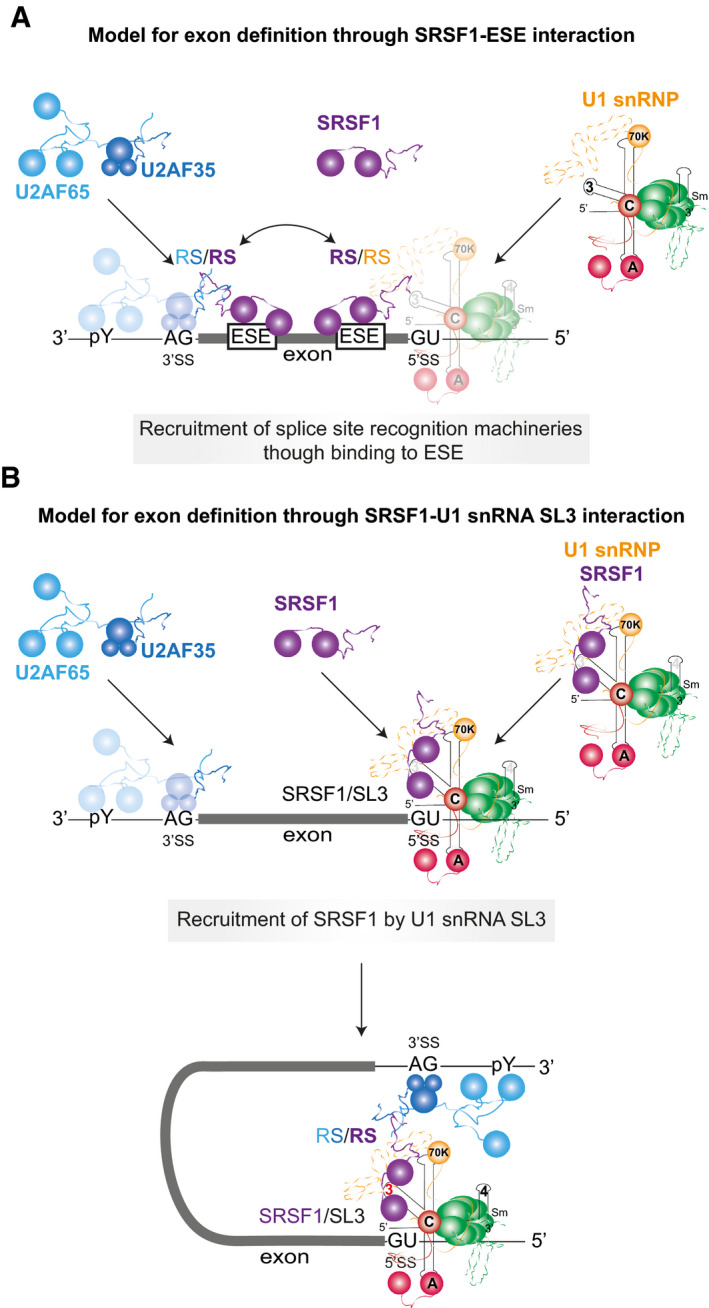
Recruitment of SRSF1 by interaction with U1 SL3 (this paper) may enable cross‐exon interactions (exon definition) and cross‐intron interactions. SRSF1 is not recruited to the pre‐mRNA by its interaction with an ESE but by its interaction with the U1 snRNA, which thereby brings SRSF1 to the 5′ splice site. This recruitment of SRSF1 may enable it to enhance the use of an upstream 3′SS in the same way as it does when bound to an ESE. SRSF1 recruited by a U1 snRNP may also mediate cross‐intron interactions with the same partners but at the downstream 3′SS during splicing reactions (Wu & Maniatis,
1993). In addition, in both cross‐intron and cross‐exon configurations, U1 snRNP‐bound SRSF1 may recruit the tri‐snRNP to the intron definition or exon definition A complexes (Roscigno & Garcia‐Blanco,
1995; Schneider
et al,
2010). Competition by other proteins for SL3 might be the basis for many examples of regulation.