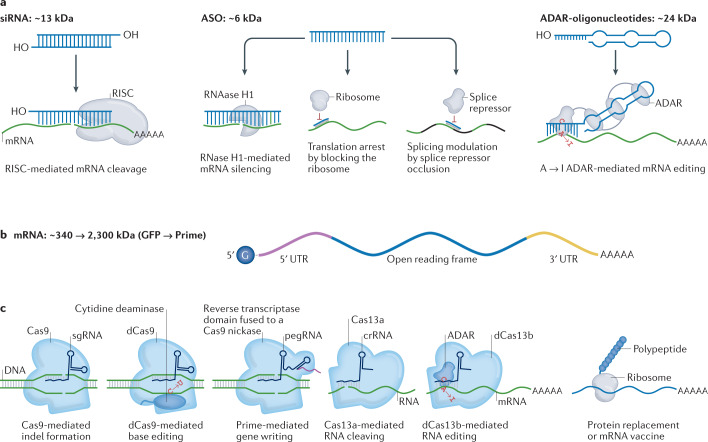
Fig. 1. The expanding universe of therapeutic RNA payloads.
a | One class of RNA therapeutics requires delivery of small RNA molecules. Small interfering RNAs (siRNAs) can reduce gene expression via RNA-induced silencing complex (RISC)-mediated mRNA degradation, antisense oligonucleotides (ASOs) can alter isoforms by binding to splice sites, and adenosine deaminase acting on RNA ASOs (ADAR-oligonucleotides) can edit RNA. In all three cases, these small RNAs can be designed with site-specific chemical modifications using solid-phase synthesis and can be delivered using nanoparticles or conjugate delivery systems. In this figure, the blue molecule represents the small therapeutic RNA being ferried into the cell. b | A second class of RNA therapeutics requires delivery of large RNA molecules. In vitro transcribed mRNA consists of a 5′ cap, 5′ and 3′ untranslated regions (UTRs), an open reading frame encoding antigen(s), and a 3′ poly(A) tail. c | mRNA payloads can encode nucleases that mediate DNA or RNA editing. mRNA can also be used to replace dysfunctional protein or encode antigens that confer longer-term immunity to a pathogen, such as SARS-CoV-2. mRNAs are transcribed in vitro and thus cannot currently be made with site-specific chemical modifications. In this figure, the blue molecule represents the protein encoded by the mRNA. Cas, CRISPR-associated protein; crRNA, CRISPR RNA; dCas9, dead Cas9; GFP, green fluorescent protein; pegRNA, prime editing guide RNA; sgRNA; single-guide RNA.