Abstract
The purpose of this study is to develop a sensitive LC-MS-MS method to simultaneously quantify polydatin and its metabolite, resveratrol, for its application in a pharmacokinetic (PK) study and to determine polydatin hydrolysis by microflora. A Shimadzu UHPLC system coupled to an AB Sciex QTrap 4000 mass spectrometer was used for the analysis. Separation was achieved using an Acquity BEH C18 column (2.1 x 50 mm) with acetonitrile and 0.1% formic acid as the mobile phases. Analysis was performed under negative ionization mode using the multiple reaction monitoring (MRM) approach. The method was linear in the range of 9.77 – 1,250 nM for both resveratrol and polydatin with correlation coefficient values >0.99. The method has been shown to be reproducible, with intra- and inter-day accuracy and precision ±10.4% of nominal values, for both analytes. The average extraction recovery rates were 81.78 to 98.3% for polydatin and 86.4 to 103.2% for resveratrol, respectively. Matrix effect was in the acceptable range (<15%). The analytes in plasma were found to be stable under bench-top, freeze-thaw, and storage (− 4°C) conditions. The metabolic studies showed that polydatin can be rapidly hydrolyzed by rat fecal S9 fractions and PK studies showed that both polydatin and resveratrol were exposed in the plasma and variable tissues. This novel UPLC-MS-MS method can quantify the levels of both polydatin and its major metabolite resveratrol in biological samples.
Keywords: polydatin, resveratrol, LC-MS, PK
1. Introduction
Polydatin, also called piceid, is a stilbene isolated from the bark of Picea sitchensis and Polygonum cuspidatum [1]. Chemically, polydatin is a glucoside of resveratrol (3,4′,5-trihydroxy-stilbene), where the glucoside group is bound to the hydroxyl at position-3 (Fig. 1). Both trans- and cis-polydatin exist, but it was reported that the trans isomer is more interesting due to its higher biological activities [2]. Polydatin is also contained in fruits, food, and beverages such as grape, peanut, hop cones, hop pellets, red wines, cocoa-containing products, chocolate products, etc. [1]. Polydatin containing cream (e.g., Sooryehan Pure-whitening cream) is sold as an over the counter (OTC) product for whitening spot treatment in the US market [3].
Figure 1.
Chemical structures of polydatin, resveratrol, and wogonin (I.S.)
Pharmacological studies have shown that polydatin possesses multiple functions including anti-inflammatory, immunoregulatory, anti-oxidative, and anti-tumor activities [1]. For example, it was reported that polydatin suppressed proliferation and metastasis of non-small cell lung cancer cells by inhibiting NLRP3 inflammasome activation via the NF-κB pathway [4]. In another example, polydatin suppressed the development of lung inflammation and fibrosis [5]. Moreover, polydatin was reported to have synergistic activity with some drugs. Some of these activities include the synergistic effect of polydatin with paclitaxel on osteosarcoma cells and that of polydatin with vitamin C in inhibiting cardiotoxicity induced by doxorubicin [6, 7]. Additionally, polydatin was reported to be active in alleviating drug induced toxicity in animal models. Examples include protection of acetaminophen-induced hepatotoxicity via the anti-oxidative pathway, protection of radiation-induced injury in intestinal epithelial and endothelial cells, protection of cerebral ischemia, protection of ovalbumin-induced bronchial asthma, and protection of cisplatin-induced toxicity [8–12]. Due to these promising pharmacological functions, clinical studies using polydatin to treat shock, chronic pain, and IBS have been conducted [13–16].
Polydatin is a glycoside and can be hydrolyzed in the gut after oral administration to release the aglycone resveratrol, a well-known compound possessing many types of biological activities such as anti-inflammation, anti-cancer, and vascular function [17–19]. A particular concern for polydatin studies is whether the efficacy is from the glycoside (i.e., polydatin), which is difficult to be absorbed, or from the aglycone (i.e., resveratrol), which is easily absorbed. Researchers have even reported that polydatin and resveratrol can be converted mutually in-vivo [20], a highly unusual property. Therefore, it is necessary to quantify both polydatin and resveratrol in in vivo studies to fully evaluate efficacy.
Quantification of drugs and their metabolites in biological samples (e.g., blood tissues) is challenging because their concentrations are usually low, and the matrix is complex that may interfere with the analytes. Liquid chromatography (LC) coupled with mass spectrometry (MS) LC-MS is a powerful tool in pharmaceutical analysis due to its high sensitivity and high specificity[21]. Novel technologies are continuously applied to improve the performance of LC-MS in the past decades, resulting in increasing applications of LC-MS in drug development. However, the procedure of LC-MS analysis is complicated, and the sensitivity and specificity of an LC-MS method could be significantly affected by many factors including type of mobile phases and columns, MS compound and instrument dependent parameters, and sample process. To ensure the reliability, an LC-MS method must be validated before being applied in biological analysis.
Analytical methods using LC-MS to quantify polydatin and resveratrol are available to quantify both polydatin and resveratrol in plant extracts and food products. For example, an LC-MS method has been reported to simultaneously quantify polydatin and resveratrol from wine [22]. However, a sensitive and robust analytical method using LC-MS to quantify these two analytes in biological samples (e.g., plasma) has not been reported and validated. In this paper, we developed and validated a sensitive LC-MS method to simultaneously quantify polydatin and resveratrol and applied the method in a PK study using rats.
2. Materials and methods
2.1. Chemicals
The standard compound polydatin, resveratrol, and formononetin (I.S) were purchased from Toronto Research Chemicals (Toronto, Canada, all compounds purity ≥ 99%). LC-MS grade acetonitrile and water were from EMD (Gibbstown, NJ, USA). Other chemicals were used as received.
2.2. Instrument and conditions.
Separation was achieved using an Acquity UPLC BEH C18 column (2.1 × 50 mm) in a Shimadzu Ultra performance liquid chromatography (UPLC) system. Acetonitrile and 0.1% formic acid were used as the mobile phase A and mobile phase B, respectively. The elution gradient was as follow: 0–0.5 min (95–95% B), 0.5–2.0 min (95–65% B), 2.0–4.0 min (65–5% B), 4.0–5.0 min (5–5% B), 5.0–5.2 min (5–95% B), and 5.2–6.0 min (95–95% B). The flow rate was 0.4 mL/min. The temperature for the auto-sampler and the column was 10 °C and 40 °C, respectively.
The analyte was quantified using an AB Sciex QTrap 4000 mass spectrometer equipped with an electro-spray ionization (ESI) source. Multiple reactions monitoring (MRM) scan type was used in the negative scan mode to increase the specificity of the analysis. The system was: ion source temperature was 400 °C; nebulizer gas(gas1), nitrogen, 30 psi; turbo gas (gas2), nitrogen 25 psi; curtain gas, nitrogen 30 psi. Unit mass resolution was set in both mass-resolving quadruples Q1 and Q3.
2.3. Preparation of stock solution, working solution, calibration curve in plasma, quality control (QC) samples, and PK samples.
The stock solutions of polydatin and resveratrol (10 mM) were prepared in 50% acetonitrile by accurately weighing appropriate amounts of polydatin and resveratrol and dissolving the powder into the solvent in a volumetric flask. Working solutions were prepared by diluting the stock solution of polydatin and resveratrol into 50% acetonitrile at final concentrations of 5,000.00, 2500.00, 1,250.00, 625.00, 312.50, 156.25, 78.13, 39.06, 19.53, 9.77, 4.88, and 2.44 nM.
The standard curve samples in plasma were prepared by spiking each of the above working solutions (20 µL) into blank plasma (20 µL) and extracting with 200 µL of acetonitrile containing 0.1µM of formononetin as the internal standard (I.S.). After centrifugation at 14,000 ×g for 15 min at 4 °C, the supernatant was transferred into a clean microcentrifuge tube and the solvent was evaporated under N2 flow. The residue was re-constituted in 50% acetonitrile (100 µL) for injection after centrifugation (20,000 ×g, 15 min, and 4 °C). The QC samples were prepared at 20 (Low, L), 400 (Medium, M), and 800 nM (High, H) following the same procedure.
The PK plasma samples were prepared by spiking blank solvent (50% acetonitrile, 20 µL) into the plasma samples (20 µL) and extracting using 200 µL of acetonitrile containing I.S., as described above.
2.4. Method validation.
The method was validated according to the bioanalytical method validation guidelines of the FDA by evaluating the specificity, linearity, recovery, matrix effect, accuracy, precision, and stability.
2.4.1. Linear range.
The calibration curve samples were prepared following the protocols described in section 2.3. Linearity was determined by plotting the peak area ratio of polydatin and resveratrol to formononetin (I.S.). Least-squares linear regression method (1/x2 weight) was used to determine the slope, intercept, and correlation coefficient of the linear regression equation. The lower limit of detection (LLOD) was defined based on a signal-to-noise ratio of 10:1.
2.4.2. Extraction Recovery and matrix effect.
The extraction recovery was determined by comparing the relative peak areas obtained from blank plasma spiked with analytes to those obtained from water spiked with the same amount of analytes. Matrix effect was determined by comparing the peak areas of blank plasma extracts spiked with analytes and I.S. to those of the standard solutions dried and reconstituted in the mobile phase. These evaluations were performed according to the recommended validation procedures reported previously [23].
2.4.3. Accuracy and precision.
The accuracy was calculated using QC samples at L, M, and H concentrations. The intra-day and inter-day precision was determined by analyzing the QC samples at these three concentrations injected on the same day and on three consecutive days.
2.4.4. Stability.
The freeze-thaw stability was evaluated by performing three freeze-thaw cycles of QC samples at L, M, and H concentrations. Samples were stored at −80 °C for 24 hours and thawed unassisted at room temperature, then were refrozen and re-thawed for a total of three cycles. The bench-top stabilities were evaluated by maintaining the QC samples at 25 °C for 2 and 4 h before analyzing. The long-term stability was determined by storing the QC samples for 14 days at −80 °C.
2.5. Fecal S9 fraction hydrolysis.
Polydatin hydrolysis by intestinal microflora was determined using fecal S9 fraction, following the protocol published by us previously [24]. Briefly, fresh feces collected from rats were pooled, washed by cold KPI buffer, sonicated, and centrifuged at 9,000 × g at 4 °C for 30 min. The supernatant was then collected as fecal S9 fraction. To determine the hydrolysis rates, different concentrations of polydatin were incubated with 0.1 mM fecal S9 fraction for 2 hours, after which the reaction was terminated using 6% acetic acid in acetonitrile. Samples were prepared and resveratrol concentrations were determined in the reaction system.
A UPLC method was used to quantify polydatin and resveratrol. The separation was achieved using an Acquity UPLC BEH C18 column (2.1 × 50 mm) in a Waters Acquity UPLC system with PDA detector. Acetonitrile and 0.1% formic acid were used as the mobile phase A and mobile phase B, respectively. The elution gradient was as follow: 0–0.5 min (95–95% B), 0.5–2.0 min (95–65% B), 2.0–4.0 min (65–5% B), 4.0–5.0 min (5–5% B), 5.0–5.2 min (5–95% B), and 5.2–6.0 min (95–95% B). The flow rate was 0.4 mL/min. The temperature for the auto-sampler and the column was 10 °C and 40 °C, respectively.
2.5. Pharmacokinetic study using SD rats.
Male F344 rats (200–250 g) were obtained from Charles River Laboratories (Wilmington, MA). Animals were maintained in a controlled environment animal facility (22 ± 2°C and 55 ± 5% relative humidity on a 12 h light/12 h dark cycle) for 10 days prior to the experiment. Before PK studies, the rats were fasted overnight with free access to water. Polydatin (100 mg/kg) was administered through oral gavage. Blood samples (50 ~ 80 μL) were collected into heparinized microcentrifuge tubes from the fossa orbitalis vein at 0, 0.25, 0.5, 1, 2, 4, 8, and 24 hours after gavage and stored at −80°C until analysis. The pharmacokinetic parameters were calculated with WinNonlin 6.3 software, using non-compartment model.
3. Results and Discussion
3.1. LC-MS Condition Optimization
Resveratrol is the aglycone of polydatin. To avoid cross peaks, we separated the two analytes using a gradient elution on a C18 column. Methanol, acetonitrile, 2.5 mM ammonia acetate (pH = 7.6), 0.1–0.5 % formic acid, and 100% water were tested as potential mobile phases. A gradient elution was established as described in section 2.2. The results showed that peak resolution and peak shape were the best when eluted with 0.1% formic acid and acetonitrile.
For MS conditions, both positive and negative scan modes were evaluated. According to the intensity of polydatin and resveratrol, negative scan was selected. Multiple reaction monitoring (MRM) scan type was used to improve the specificity. The compounds and instrument dependent parameters were optimized by tuning polydatin and resveratrol separately. The compound dependent MS parameters are shown in Table 1. A represented chromatogram is shown in Fig. 3 and the MS/MS spectra of polydatin, resveratrol, and I.S. are shown in Fig. 2.
Table 1.
Compound -dependent parameters for polydatin and resveratrol in MRM mode of UPLC-MS/MS analysis.
| Analytes | Q1 (m/z) | Q3(m/z) | Dwell time (ms) | DP (V) | EP (V) | CE (V) | CXP (V) |
|---|---|---|---|---|---|---|---|
| Polydatin | 389 | 227 | 200 | −39.0 | −10 | −18.0 | −3.0 |
| Resveratrol | 227 | 185 | 200 | −53.0 | −10 | −26.0 | −9.0 |
| Wogonin (I.S.) | 283 | 162 | 200 | −80.0 | −10 | −40.0 | −15.0 |
DP: Declustering potential, EP: Entrance Potential; CE: Collision energy, CXP: Collision cell exit potential
Figure 3.
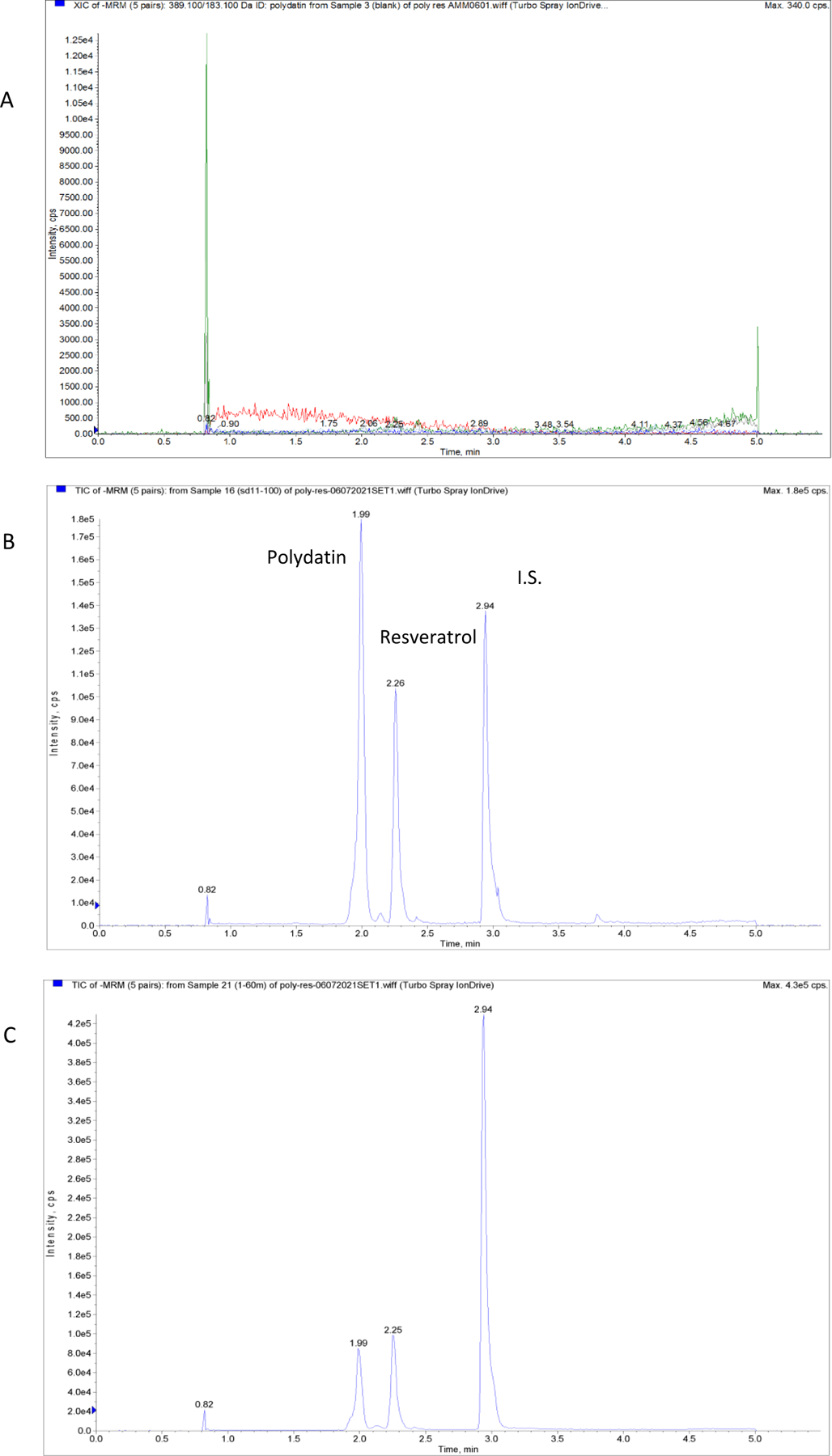
Representative MRM chromatograms of blank plasma (A), blank plasma spiked with polydatin and resveratrol (B), and a PK sample at 60 min (C).
Figure 2.
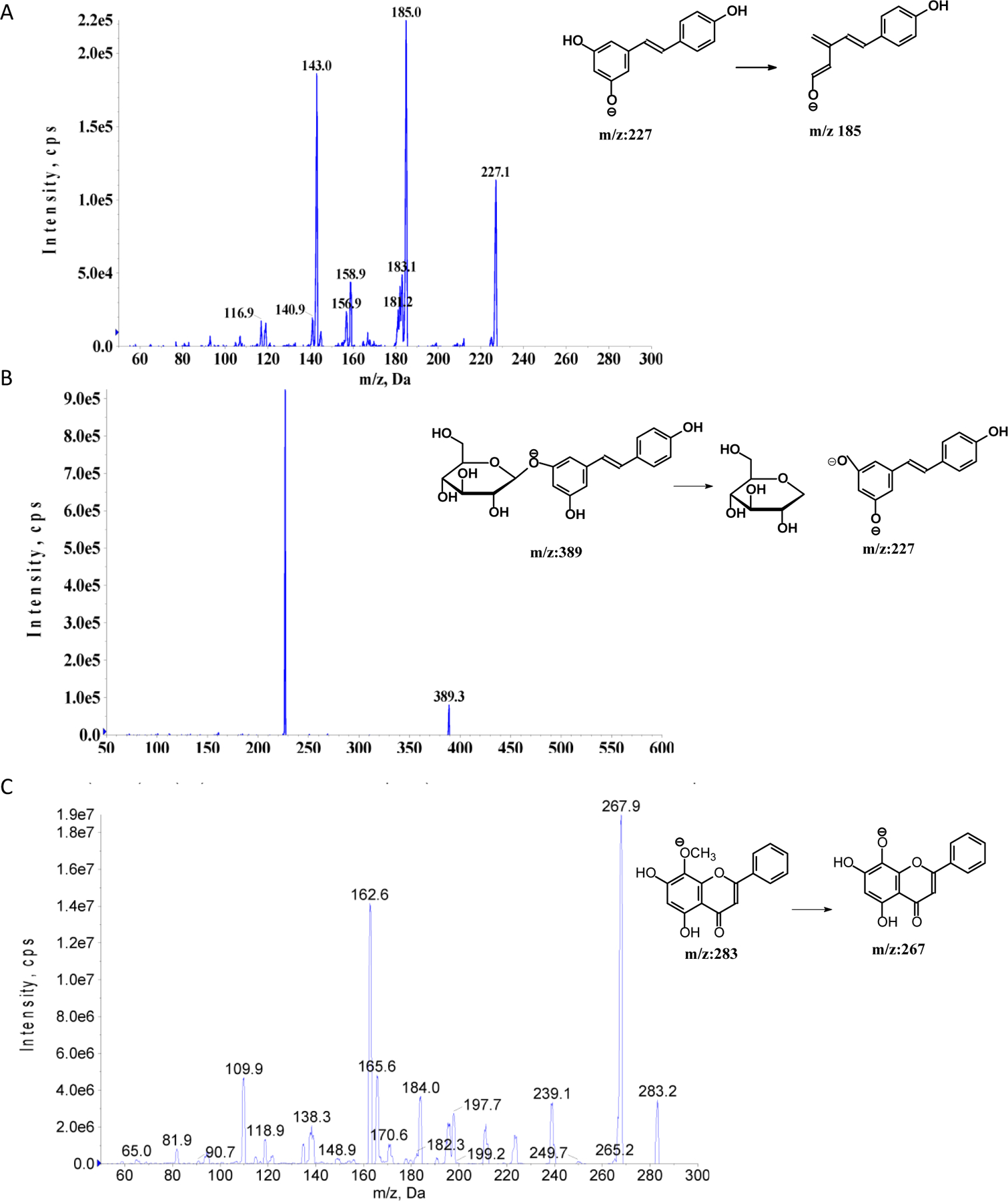
MS/MS spectra of polydatin, resveratrol, and wogonin (I.S.)
3.2. Method validation
3.2.1. Linearity
The linear range was determined in blank plasma. The developed method was linear in the range of 9.77 – 1,250 nM for both resveratrol and polydatin. Calibration curves resulted in R2 values ≥ 0.99. The LLOD was 2.44 nM for both polydatin and resveratrol.
3.2.2. Accuracy and precision
Accuracy and precision results for QC concentrations prepared in rat plasma are shown in Table 2. Polydatin intra- and inter-day results averaged 100.2%, resveratrol results averaged 101.4%. The accuracy and precision were found to be within the acceptable range for both analytes.
Table 2.
Extraction Recovery, Matrix effect, Precision, and Accuracy of polydatin and resveratrol in MRM mode of UPLC-MS/MS analysis
| Extraction recovery | Matrix effect | Precision |
||||||
|---|---|---|---|---|---|---|---|---|
| Analyte | QC samples | Intraday (n=6) | Interday (n=6) | |||||
|
|
||||||||
| Mean (%) | SD | RSD (%) | Precision (RSD, %) | Accuracy (%) | Precision (RSD, %) | Accuracy (%) | ||
| polydatin | L | 81.78 | 6.68 | 98.4 | 8.83 | 100.10 | 7.86 | 100.7 |
| M | 98.30 | 9.30 | 89.5 | 5.81 | 99.20 | 5.77 | 97.9 | |
| H | 94.70 | 14.00 | 96.1 | 3.93 | 102.26 | 3.53 | 102.0 | |
| Resveratrol | L | 103.20 | 10.54 | 89.8 | 4.29 | 100.2 | 8.08 | 101.1 |
| M | 86.40 | 7.50 | 95.2 | 6.24 | 97.02 | 9.14 | 99.5 | |
| H | 91.40 | 9.05 | 95.0 | 4.29 | 102.8 | 5.11 | 103.5 | |
3.2.3. Recovery and matrix effect
The average extraction recovery rates and matrix effect obtained by measuring triplicates of QC samples at low, medium, and high concentrations of polydatin and resveratrol in rat plasma are shown in Table 2. The extraction recovery for these three analytes ranged from 81.3 ± 4.1% to 113.9 ± 13.2%. There was no significant matrix effect and no significant degradation under experimental conditions.
3.2.4. Plasma stability
The analyte mixture in plasma was found to be stable under bench-top, freeze-thaw, and storage (− 4° C) conditions. The bias of short-term bench-top stability of polydatin averaged 99.4% and resveratrol stability averaged 102.3%. The bias of freeze thaw stability of polydatin averaged 99.89%, resveratrol stability averaged 103.1% (Table 3).
Table 3.
Stability of polydatin and resveratrol in rat plasma under different storage conditions
| QC samples | 25℃ for 4h | Three-freeze-thaw cycles | Long term storage | ||||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| Analytes | Stability (%) | CV(%) | Stability (%) | CV(%) | Stability (%) | CV(%) | |
| Polydatin | L | 99.72 | 10.37 | 95.37 | 6.89 | 102.4 | 10.6 |
| M | 99.08 | 6.99 | 102.2 | 7.33 | 99.20 | 12.6 | |
| H | 99.42 | 1.75 | 102.1 | 6.18 | 102.3 | 14.2 | |
| Resveratrol | L | 104.6 | 7.22 | 104.8 | 6.44 | 98.54 | 7.75 |
| M | 97.72 | 6.37 | 106.9 | 5.69 | 103.7 | 7.56 | |
| H | 104.6 | 6.89 | 97.68 | 6.29 | 103.3 | 7.02 | |
3.3. Polydatin hydrolysis to release resveratrol.
Resveratrol was identified as the hydrolysis product using the established LC-MS-MS method. The hydrolysis kinetic parameters were calculated and are listed in Fig. 4. The results showed the Km to be 77.25 ± 5.95 nM and the Vmax was 2,573 ± 85.99 nmol/mg/min.
Figure 4.
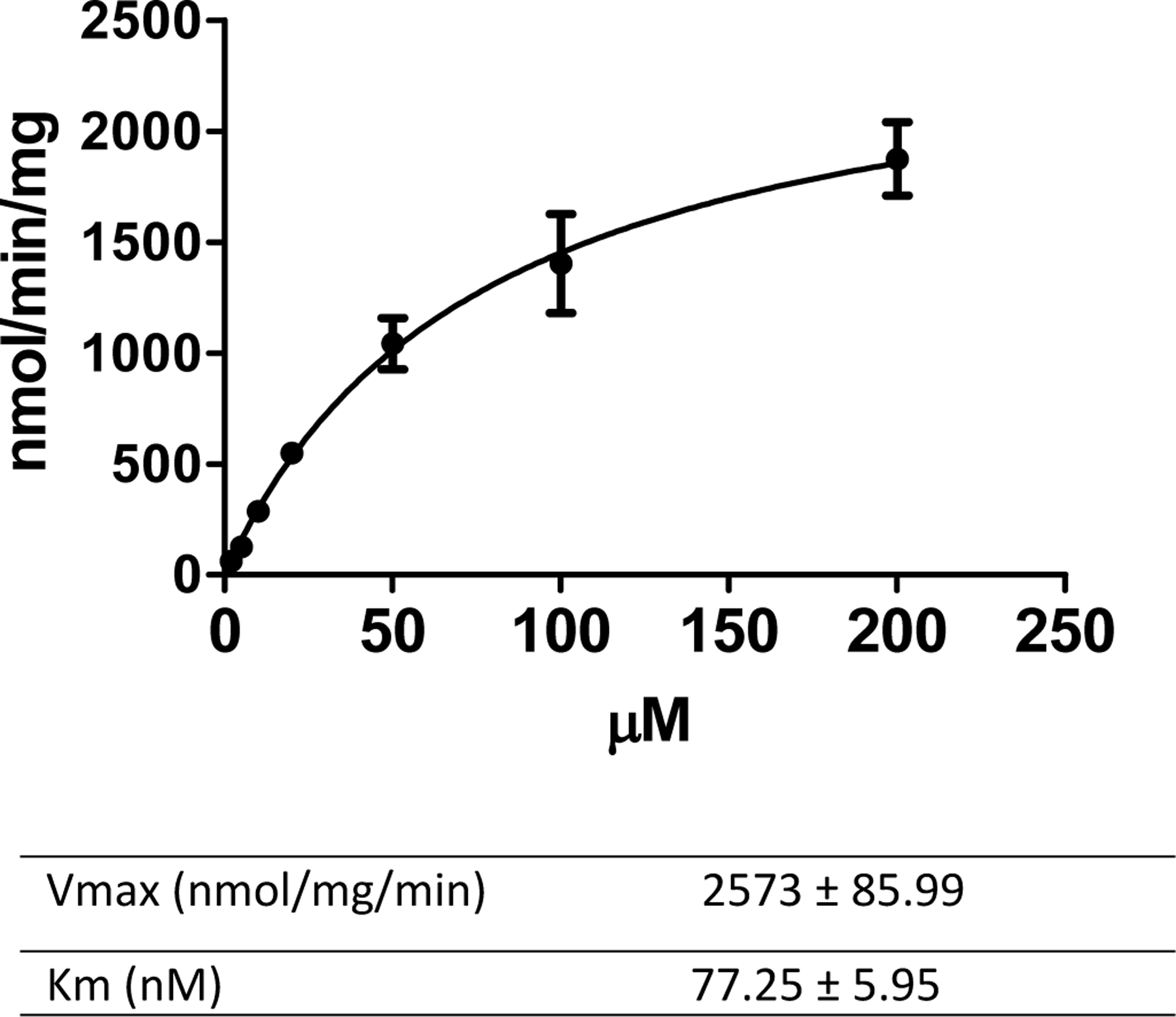
Polydatin hydrolysis using enzymes prepared from rat feces. The reaction was carried out for 60 min at 37°C.
3.4. Application in a PK study is rats.
The mean plasma concentration-time curves of polydatin and resveratrol are presented in Fig. 5 and the PK parameters are listed in Table 4. The results showed that the concentration of polydatin in the plasma decreased rapidly, with a terminal half-life (t1/2) of 1.02 ± 0.08 h. At the current dose, the Cmax and AUC0-t of polydatin were 1.43 ± 0.36 μM and 1.42 ± 0.42 μmol h/L, respectively. Interestingly, high exposure of resveratrol was detected in the plasma. The Cmax and AUC0-t of resveratrol was 1.36 ± 0.47 μM and 1.70 ± 0.14 μmol h/L, respectively. These results indicate that when polydatin is administered through the oral route, a large proportion of the parent compound is converted into resveratrol. This conclusion was further supported by in-vitro polydatin hydrolysis via fecal S9, in which rapid hydrolysis was observed.
Figure 5.
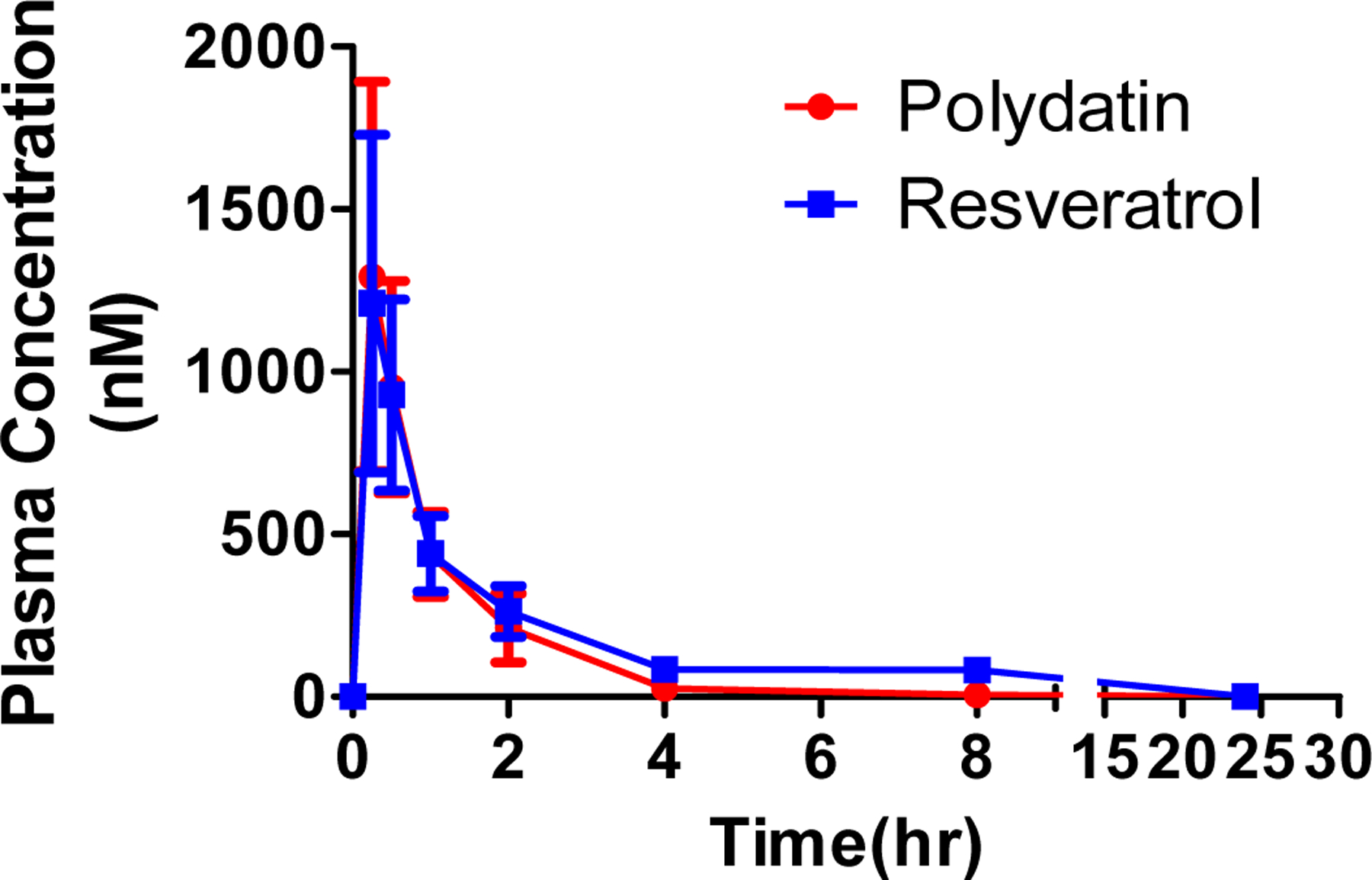
Plasma concentration versus time profiles of polydatin and its metabolite resveratrol after oral administration of polydatin. Each symbol represents the mean and the error bars indicate the S.D.
Table 4.
Pharmacokinetic parameters of polydatin and resveratrol after oral administration of polydatin (20 mg/kg) to rat (n=4)
| Parameters | Polydatin | Resveratrol |
|---|---|---|
| Tmax(h) | 0.33 ± 0.11 | 0.33 ± 0.12 |
| Cmax(μg/L) | 0.56 ± 0.14 | 0.36 ± 0.11 |
| AUC0~t(μg h/L) | 0.55 ± 0.16 | 0.39 ± 0.04 |
| MRT(h) | 1.16 ± 0.07 | 1.82± 0.47 |
| T1/2(h) | 1.02± 0.08 | 1.74 ± 0.58 |
| CL(L/hr/kg) | 201.67 ± 54.10 | 136.7± 3.42 |
| Vz(L/kg) | 304.01 ± 68.85 | 344.3± 114.65 |
4. Conclusions
This novel UPLC-MS-MS method for quantification of polydatin and its microbial metabolite, resveratrol, in plasma has been fully validated and can be used to simultaneously quantify both compounds in in-vivo studies. High exposure levels of resveratrol were detected in the plasma following administration of polydatin in a pharmacokinetic study in rats. This was most probably due to microbial hydrolysis, which is further supported by the results of the S9 fecal fraction assay. These findings indicate special attentions should be paid in future polydatin pharmacological efficacy studies.
An UPLC-MS/MS method to quantify polydatin and resveratrol was developed and validated
Polydatin hydrolyzes by microflora was determined
The sensitive and robust method was used in a PK study in rats
Acknowledgement
This work was supported by a grant from the Cancer Prevention Research Institute of Texas (CPRIT, RP190672) and National Institute of General Medical Sciences (1R15GM126475–01A1) for Song Gao. This work was also made possible, in part, by services provided from GCC Center for Comprehensive PK/PD and Formulation (CCPF) with CPRIT grant number of RP180748 and National Institute of Minority Health and Health Disparity (U54MD007605) for Dong Liang.
Abbreviations
- UHPLC
ultra high performance liquid chromatography
- IS
internal standard
- AUC
area under the curve
- QC
quality control
- LLOQ
lower limit of quantification
- PK
pharmacokinetic
- FDA
federal drug administration
- LLOD
lower limit of detection
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References:
- [1].Du QH, Peng C, Zhang H, Polydatin: a review of pharmacology and pharmacokinetics, Pharm Biol, 51 (2013) 1347–1354. [DOI] [PubMed] [Google Scholar]
- [2].Mikulski D, Molski M, Quantitative structure-antioxidant activity relationship of trans-resveratrol oligomers, trans-4,4’-dihydroxystilbene dimer, trans-resveratrol-3-O-glucuronide, glucosides: trans-piceid, cis-piceid, trans-astringin and trans-resveratrol-4’-O-beta-D-glucopyranoside, Eur J Med Chem, 45 (2010) 2366–2380. [DOI] [PubMed] [Google Scholar]
- [3].Drugbank, https://www.drugbank.ca/drugs/DB11263.
- [4].Zou J, Yang Y, Yang Y, Liu X, Polydatin suppresses proliferation and metastasis of non-small cell lung cancer cells by inhibiting NLRP3 inflammasome activation via NF-kappaB pathway, Biomed Pharmacother, 108 (2018) 130–136. [DOI] [PubMed] [Google Scholar]
- [5].Tang J, Li Y, Wang J, Wu Q, Yan H, Polydatin suppresses the development of lung inflammation and fibrosis by inhibiting activation of the NACHT domain-, leucine-rich repeat-, and pyd-containing protein 3 inflammasome and the nuclear factor-kappaB pathway after Mycoplasma pneumoniae infection, J Cell Biochem, 120 (2019) 10137–10144. [DOI] [PubMed] [Google Scholar]
- [6].Zhao W, Chen Z, Guan M, Polydatin enhances the chemosensitivity of osteosarcoma cells to paclitaxel, J Cell Biochem, 120 (2019) 17481–17490. [DOI] [PubMed] [Google Scholar]
- [7].Wang HL, Cui XH, Yu HL, Wu R, Xu X, Gao JP, Synergistic effects of polydatin and vitamin C in inhibiting cardiotoxicity induced by doxorubicin in rats, Fundam Clin Pharmacol, 31 (2017) 280–291. [DOI] [PubMed] [Google Scholar]
- [8].Li L, Zhang K, Zhang J, Zeng YN, Lai F, Li G, Ma N, Hu MJ, Cui FM, Chen Q, Protective effect of polydatin on radiation-induced injury of intestinal epithelial and endothelial cells, Biosci Rep, 38 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Liu YH, Huang QH, Wu X, Wu JZ, Liang JL, Lin GS, Xu LQ, Lai XP, Su ZR, Chen JN, Polydatin protects against acetaminophen-induced hepatotoxicity in mice via anti-oxidative and anti-apoptotic activities, Food Funct, 9 (2018) 5891–5902. [DOI] [PubMed] [Google Scholar]
- [10].Tang KS, Tan JS, The protective mechanisms of polydatin in cerebral ischemia, Eur J Pharmacol, 842 (2019) 133–138. [DOI] [PubMed] [Google Scholar]
- [11].Hanna DA, Khalaf MM, Abo-Saif AA, Polydatin protects against ovalbumin-induced bronchial asthma in rats; involvement of urocortin and surfactant-D expression, Immunopharmacol Immunotoxicol, 41 (2019) 403–412. [DOI] [PubMed] [Google Scholar]
- [12].Ince S, Arslan Acaroz D, Neuwirth O, Demirel HH, Denk B, Kucukkurt I, Turkmen R, Protective effect of polydatin, a natural precursor of resveratrol, against cisplatin-induced toxicity in rats, Food Chem Toxicol, 72 (2014) 147–153. [DOI] [PubMed] [Google Scholar]
- [13].Cremon C, Stanghellini V, Barbaro MR, Cogliandro RF, Bellacosa L, Santos J, Vicario M, Pigrau M, Alonso Cotoner C, Lobo B, Azpiroz F, Bruley des Varannes S, Neunlist M, DeFilippis D, Iuvone T, Petrosino S, Di Marzo V, Barbara G, Randomised clinical trial: the analgesic properties of dietary supplementation with palmitoylethanolamide and polydatin in irritable bowel syndrome, Aliment Pharmacol Ther, 45 (2017) 909–922. [DOI] [PubMed] [Google Scholar]
- [14].Murina F, Graziottin A, Felice R, Radici G, Tognocchi C, Vestibulodynia: synergy between palmitoylethanolamide + transpolydatin and transcutaneous electrical nerve stimulation, J Low Genit Tract Dis, 17 (2013) 111–116. [DOI] [PubMed] [Google Scholar]
- [15].Lo Monte G, Soave I, Marci R, [Administration of micronized palmitoylethanolamide (PEA)-transpolydatin in the treatment of chronic pelvic pain in women affected by endometriosis: preliminary results], Minerva Ginecol, 65 (2013) 453–463. [PubMed] [Google Scholar]
- [16].Tartaglia E, Armentano M, Giugliano B, Sena T, Giuliano P, Loffredo C, Mastrantonio P, Effectiveness of the Association N-Palmitoylethanolamine and Transpolydatin in the Treatment of Primary Dysmenorrhea, J Pediatr Adolesc Gynecol, 28 (2015) 447–450. [DOI] [PubMed] [Google Scholar]
- [17].Nunes S, Danesi F, Del Rio D, Silva P, Resveratrol and inflammatory bowel disease: the evidence so far, Nutr Res Rev, 31 (2018) 85–97. [DOI] [PubMed] [Google Scholar]
- [18].Vervandier-Fasseur D, Latruffe N, The Potential Use of Resveratrol for Cancer Prevention, Molecules, 24 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Dull AM, Moga MA, Dimienescu OG, Sechel G, Burtea V, Anastasiu CV, Therapeutic Approaches of Resveratrol on Endometriosis via Anti-Inflammatory and Anti-Angiogenic Pathways, Molecules, 24 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Wang HL, Gao JP, Han YL, Xu X, Wu R, Gao Y, Cui XH, Comparative studies of polydatin and resveratrol on mutual transformation and antioxidative effect in vivo, Phytomedicine, 22 (2015) 553–559. [DOI] [PubMed] [Google Scholar]
- [21].Beccaria M, Cabooter D, Current developments in LC-MS for pharmaceutical analysis, Analyst, 145 (2020) 1129–1157. [DOI] [PubMed] [Google Scholar]
- [22].Mark L, Nikfardjam MS, Avar P, Ohmacht R, A validated HPLC method for the quantitative analysis of trans-resveratrol and trans-piceid in Hungarian wines, J Chromatogr Sci, 43 (2005) 445–449. [DOI] [PubMed] [Google Scholar]
- [23].Matuszewski BK, Constanzer ML, Chavez-Eng CM, Strategies for the assessment of matrix effect in quantitative bioanalytical methods based on HPLC-MS/MS, Anal Chem, 75 (2003) 3019–3030. [DOI] [PubMed] [Google Scholar]
- [24].Niu T, Smith DL, Yang Z, Gao S, Yin T, Jiang ZH, You M, Gibbs RA, Petrosino JF, Hu M, Bioactivity and bioavailability of ginsenosides are dependent on the glycosidase activities of the A/J mouse intestinal microbiome defined by pyrosequencing, Pharm Res, 30 (2013) 836–846 [DOI] [PMC free article] [PubMed] [Google Scholar]


