In a recent article [1], Quiroga argues that there is no pattern separation in the human hippocampus based on data from human single-neuron studies. Quiroga argues instead that the hippocampus codes memories by coactivation of invariant and context-independent engrams. We believe that both pattern separation and completion are important components of human memory, and that overlooked empirical studies and theoretical considerations fundamentally challenge the arguments posed.
To clarify, definitions of episodic memory typically involve a multidimensional cognitive system that associates numerous unique details involved in an experience [2]. A computational process that differentiates overlapping inputs into more distinct codes (e.g., pattern separation) would be valuable, if not essential, to avoiding interference from the myriad of competing experiences [3]. Quiroga argues that generalization (pattern completion) is essential to memory. Of course, these same models argue for pattern completion existing in parallel as part of a ‘complementary learning systems’ division of labor. On its own, a system designed for generalization may be poorly suited to overcoming interference and unable to process specific task demands that are likely to arise within similar contexts in which memories are formed.
Screening of Human Neuronal Responses and Lack of Behavioral Assessment
Certain aspects of current human single-neuron data stand in the way of being able to claim that pattern separation does not exist in the hippocampus. First and foremost, an absence of evidence is not the same as evidence of absence. While Quiroga argues that no neurons showing pattern separation have been observed, there are reasons why these data are biased to not reveal such signals. In nearly all studies that record concept cells, a screening procedure is utilized prior to the behavioral task to identify responsive cells. While ‘screening sessions’ serve the purpose of identifying images that provoke strong neural responses [4], the selected cells are biased by the participant’s interest and expertise, leading to responses that may be more likely to show pattern completion especially in the absence of task demands. Importantly, the behavioral tasks used in concept cell studies discussed by Quiroga have no need for mnemonic discrimination of competing stimuli, a key aspect of pattern separation. Together, this screening and lack of relevant behavioral assessment may lead to an over-representation of generalization and an under-representation, or even the entire omission, of pattern separation signals in the data. Future studies using memory tasks that require discrimination of similar stimuli ([5]; for example, Figure 1) will be better suited to detect context-dependent coding in human single neurons.
Figure 1. Example of a Task Requiring Differentiation of Similar Stimuli.
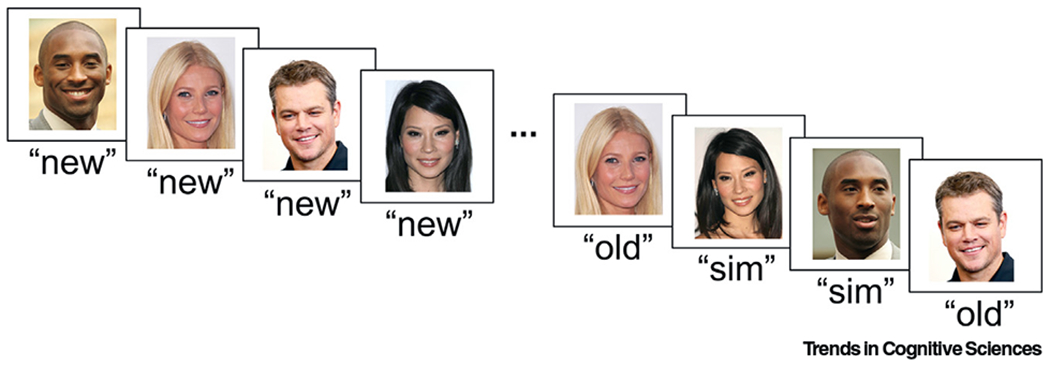
Here, we show example image stimuli that could be used in a mnemonic discrimination task in order to investigate the presence of pattern separation mechanisms in human single-neuron activity (adapted from [5]). During the task, participants would view novel images (‘new’) followed by a randomized interleaved set of the same images repeated (‘old’) and similar (‘sim’) lure images. Critically, task instructions would require participants to differentiate between image types by responding ‘old’ to repeated images and ‘sim’ to new lure images. Single-neuron activity (firing rate and/or other activity-related patterns) could then be compared between image types (‘new’, ‘old’, ‘sim’) and between successfully (correct ‘sim’) and unsuccessfully (incorrect ‘sim’) remembered trials.
Evidence Supportive of Pattern Separation
Evidence from studies not considered by Quiroga support a role for the human hippocampus in pattern separation. One study, using the same screening approach, found that human hippocampal neuronal responses were stimulus selective rather than invariant on a task that required mnemonic discrimination between similar stimuli [6]. Another study observed conjunctive responses in human single neurons during learning of spatial locations, goals, and landmarks [7]. As Quiroga notes, however, such conjunctive responses provide important evidence for context-dependent coding, and, therefore, such findings are instead consistent with neural codes important to episodic memory and pattern separation mechanisms within the human hippocampus [6]. While early fMRI evidence for pattern separation could be interpreted as a binary novelty or mismatch signal [8], later work demonstrated a nonlinear transfer function in CA3 and dentate gyrus (DG) across a range of stimulus similarity [9], consistent with pattern separation. In addition, high-resolution multivariate fMRI studies have identified pattern-separated representations in CA3 and DG, including for novel spatial environments and room configurations [10,11]. Collectively, these studies involve a continuous measure of similarity, and, thus, ‘separation’ effects cannot be attributed to a binary novelty signal.
Hippocampal Subfield Differences
Finally, models that emphasize pattern separation treat hippocampal subfields separately. Hippocampal pattern separation, if present, would be most apparent in the DG and CA3 (areas identified in animal studies). While hippocampal subfield localization is challenging, there are methods that show promise [6]. Although obstacles remain in ensuring that recordings are subfield specific, this approach will be needed to convincingly argue evidence against pattern separation in the hippocampus. Further, sparse coding in the DG makes any random sampling of hippocampal activity unlikely to have DG neurons, and evidence of a lack of pattern separation in the DG would be required to support Quiroga’s larger conclusion. Notably, a study of a patient with a localized DG lesion noted deficits in the ability to discriminate lure stimuli from targets, one of the hallmarks of pattern separation deficits [12].
In summary, while investigations of interspecies differences that explain the human brain’s unique cognitive abilities are important, evidence not considered by Quiroga suggests that pattern separation mechanisms do exist in the human hippocampus and that concept cells provide only a partial window into the neural mechanisms that support human memory. Ultimately, technological advances that enable single-neuron recordings from the DG and tasks that explicitly investigate relevant mechanisms without sampling biases will be better positioned to assess pattern separation mechanisms in the human hippocampus. Such methods, in our view, will provide additional evidence for pattern separation mechanisms and its involvement in human episodic memory.
Acknowledgments
This work was supported by the National Institutes of Health (NIH) National Institute of Neurological Disorders and Stroke (NINDS) (NS103802 and NS117838), the McKnight Foundation (Technological Innovations Award in Neuroscience to N.S.), and the Keck Junior Faculty Award (toN.S.). The authors thank Sonja Hiller for help with illustrations and manuscript preparation.
References
- 1.Quiroga RQ (2020) No pattern separation in the human hippocampus. Trends Cogn. Sci 24, 994–1007 [DOI] [PubMed] [Google Scholar]
- 2.Tulving E (2005) Episodic memory and autonoesis: uniquely human? In The Missing Link in Cognition: Origins of Self-Reflective Consciousness (Terrace HS and Metcalfe J, eds), pp. 3–56, Oxford University Press [Google Scholar]
- 3.McClelland JL et al. (1995) Why there are complementary learning systems in the hippocampus and neocortex: insights from the successes and failures of connectionist models of learning and memory. Psychol. Rev 102, 419–457 [DOI] [PubMed] [Google Scholar]
- 4.Suthana NA and Fried I (2012) Percepts to recollections: insights from single neuron recordings in the human brain. Trends Cogn. Sci 18, 427–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lohnas LJ et al. (2018) Time-resolved neural reinstatement and pattern separation during memory decisions in human hippocampus. Proc. Natl. Acad. Sci 115, E7418–E7427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Suthana NA et al. (2015) Specific responses of human hippocampal neurons are associated with better memory. Proc. Natl. Acad. Sci 112, 10503–10508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ekstrom AD et al. (2003) Cellular networks underlying human spatial navigation. Nature 425, 184–187 [DOI] [PubMed] [Google Scholar]
- 8.Bakker A et al. (2008) Pattern separation in the human hippocampal CA3 and dentate gyrus. Science 319, 1640–1642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lacy JW et al. (2011) Distinct pattern separation related transfer functions in human CA3/dentate and CA1 revealed using high-resolution fMRI and variable mnemonic similarity. Learn. Mem 18, 15–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stokes J et al. (2015) Complementary roles of human hippocampal subfields in differentiation and integration of spatial context. J. Cogn. Neurosci 27, 546–559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berron D et al. (2016) Strong evidence for pattern separation in human dentate gyrus. J. Neurosci 36, 7569–7579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baker S et al. (2016) The human dentate gyrus plays a necessary role in discriminating new memories. Curr. Biol 26, 2629–2634 [DOI] [PubMed] [Google Scholar]


