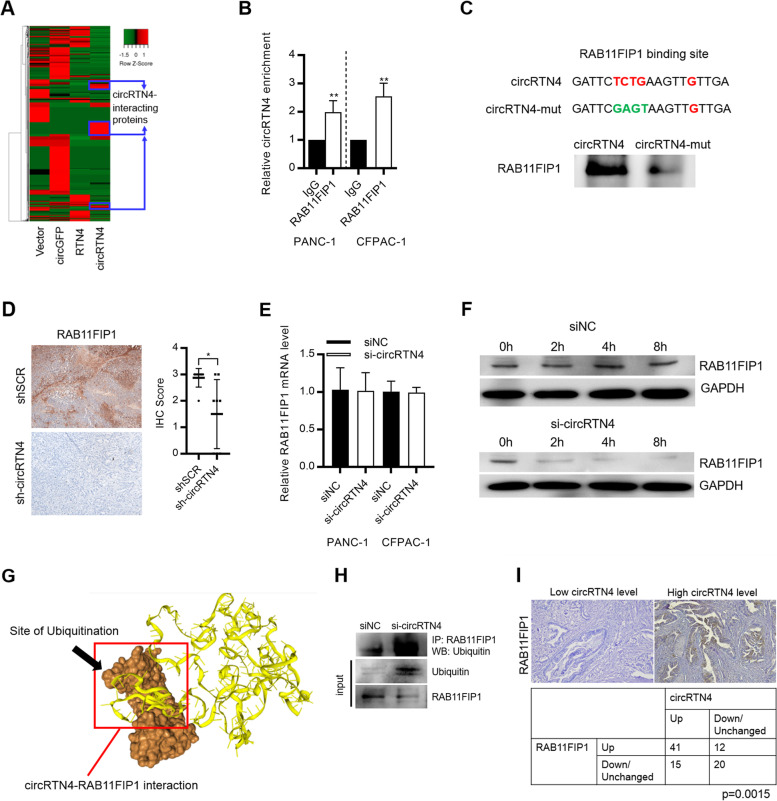
Fig. 5.
circRTN4 stabilizes RAB11FIP1 by preventing its ubiquitination and degradation. A Heat-map showing 99 circRTN4-interacting proteins in PDAC. Biotin-labelled circRTN4, RTN4 mRNA, circGFP were used to pull down circRTN4-interacting proteins. Mass spectrometry analysis was performed to identify the interacting proteins. B CircRTN4 interacted with RAB11FIP1 in PDAC cells, as revealed by RIP assay. C Bioinformatics analysis by PRIdictor revealed the RAB11FIP1-binding site (The seed region of interaction was in red) on circRTN4. Mutating RAB11FIP1-binding site on circRTN4 (The mutated seed region of interaction was in green) inhibited circRTN4-RAB11FIP1 interaction in PANC-1 cells, as revealed by circRNA pulldown assay. D CircRTN4 knockdown inhibited RAB11FIP1 expression in mice xenograft. E CircRTN4 knockdown did not affect RAB11FIP1 mRNA level in PDAC cells. F CircRTN4 knockdown decreased the stability of RAB11FIP1 after inhibition of protein synthesis by cycloheximide in PANC-1 cells. G 3-Dimensional structure of the circRTN4-RAB11FIP1 interaction revealed that circRTN4 blocked the ubiquitination site Lys578 of RAB11FIP1. H Immunoprecipitation with anti-RAB11FIP1 antibody in PANC-1 cells after circRTN4 knockdown, followed by immunoblotting analysis with anti-ubiquitin or anti-RAB11FIP1 antibody. CircRTN4 knockdown increased ubiquitination of RAB11FIP1. I RAB11FIP1 expression were upregulated in PDAC primary tumors and was positively correlated with circRTN4 level. Data represent mean ± SD from at least three independent experiments (*p < 0.05; **p < 0.01; ***p < 0.001)