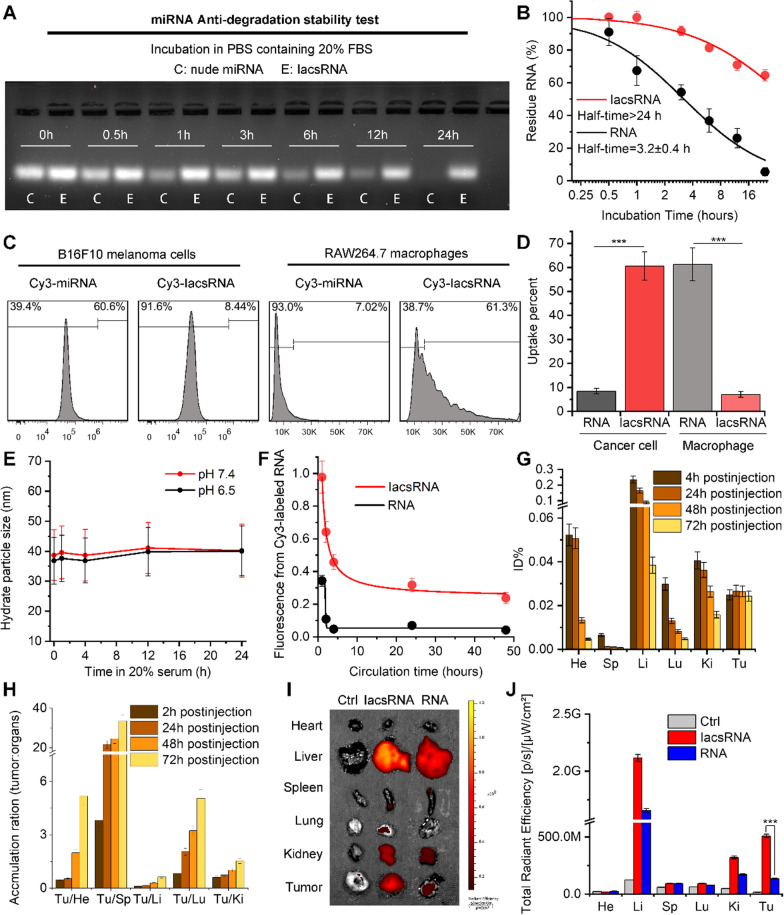
Fig. 2.
Physicochemical and pharmaceutical properties of IacsRNA. A, B Anti-degradation stability test (A) and quantitative analysis (B) of IacsRNA and nude miRNA by gel electrophoresis. C, D Flow cytometry analysis (C) and quantification (D) of different cells uptake of 1 µM Cy3-labelled miRNA and IacsRNA after 6 h incubations. (E) Colloidal stability of IacsRNA suspending in PBS containing 20% FBS at pH7.4 and 6.5 measured by DLS. F Blood-circulation curves of IacsRNA and RNA in healthy C57/B6 mice quantified by the fluorescence signal from the labeled Cy3 fluorescein in miRNA. G organ distribution of IacsRNA in BALB/c mice after systemic injection. Serial sacrifices were carried out at 2 h, 24 h, 48 h and 72 h after injection. Several organs/tissues, including heart, liver, spleen, lung, kidney and tumor were isolated to determine gold concentrations by ICP-MS. He, heart; Sp, spleen; Li, liver; Lu, lung; Ki, kidney; Tu, tumor. H Tumor-to-organ ratios for IacsRNA at 2 h, 24 h, 48 h and 72 h after injection. I, J Tissue distribution (I) and quantification (J) of IacsRNA. The fluorescence signal from the tumors and normal organ after IacsRNA intraperitoneal injection (200 µL, 1 mM Au) at 1 h. The data were presented as mean ± s.d. *, p < 0.05; **, p < 0.01; ***, p < 0.001