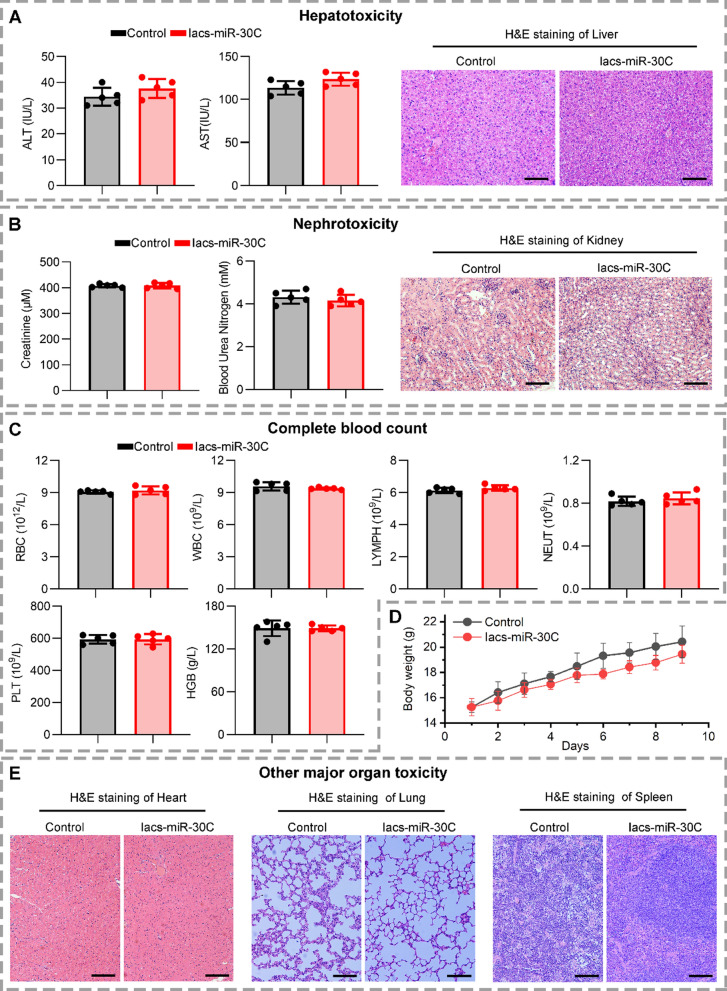
Fig. 3.
The biosafety evaluation of IacsRNA in vivo. A Hepatotoxicity testing of the IacsRNA measured by aspartate transaminase (ALT), alanine aminotransferase (AST), and pathological section of liver (scale bar: 100 μm). B Nephrotoxicity testing of the IacsRNA measured by blood urea nitrogen (BUN), creatinine (CRE) and pathological section of kidney (scale bar: 100 μm). C Analysis of red blood cell (RBC), white blood cell (WBC), lymphocyte (LYMPH), Neutrophil (NEUT), Platelets (PLT) and Hemoglobin (HGB) in mice blood with the indicated treatments. D Body weight of mice with the indicated treatments. E Toxicity testing of the IacsRNA measured by pathological section of heart, lung and spleen. The data were presented as mean ± s.d. *, p < 0.05