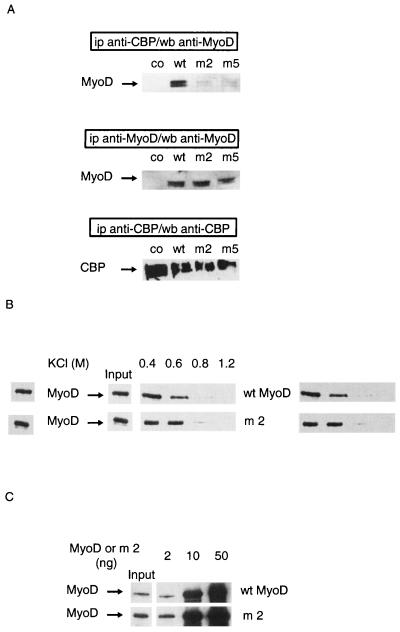
FIG. 2.
Nonacetylatable MyoD mutants do not associate with CBP in cells. Mutants used were m2, in which lysines 99, 102, and 104 were replaced by arginines, and m5, in which lysines 99, 102, 104, 112, 124, and 133 were replaced by arginines. (A) C3H 10T1/2 cells were transfected with expression vectors for wild-type (wt) or point-mutated (m2 or m5) MyoD together with an expression vector for wt CBP 1-2441. A MyoD empty vector vehicle was used in negative controls (co). Extracts from transfected cells were immunoprecipitated (ip) using an anti-CBP antibody or an anti-MyoD antibody as indicated. MyoD and CBP were detected by Western blotting (wb), as indicated. (B) Nonacetylated wt or mutant MyoD (m2) was incubated with GST-CBP-coated beads for 1 h and washed with TE buffer containing the indicated concentrations of KCl. Beads were analyzed by Western blotting using anti-MyoD antibodies. (C) Indicated doses of nonacetylated MyoD (wt or mutated) were incubated in TE buffer with GST-CBP-coated beads. Beads were analyzed by Western blotting using anti-MyoD antibodies. Input, the amount of each MyoD protein, estimated as 2 ng (1/25 of maximal amount used in this experiment).