Abstract
In this review, we present the current state of knowledge surrounding mammalian digit tip regeneration. We discuss the origin and formation of the blastema, a structure integral to digit tip regeneration, as well as recent insights driven by single-cell RNA sequencing into the molecular markers and cellular composition of the blastema. The digit tip is a composite of many different tissue types and we address what is known about the role of these separate tissues in regeneration of the whole digit tip. Specifically, we discuss the most extensively studied tissues in the digit tip: bone, nail epithelium, and peripheral nerves. We also address how known molecular pathways in limb development can inform research into digit tip regeneration. Overall, the mouse digit tip is an excellent model of complex mammalian regeneration that can provide insight into inducing regeneration in human tissues.
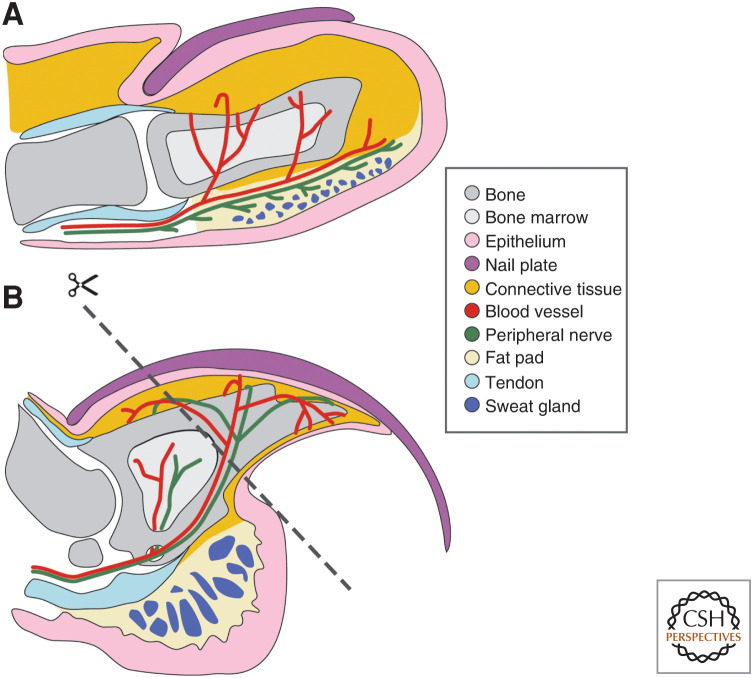
Regenerative abilities vary dramatically across the animal kingdom and even within the vertebrate lineage. On one extreme, the highly regenerative axolotl is capable of regenerating limbs, the tail, the spinal cord, and other complex tissues, and zebrafish can regenerate their fins and hearts after injury (Johnson and Weston 1995; Poss et al. 2002). On the other hand, regenerative ability in mammals is limited and generally restricted to regeneration of an organ such as the liver (Higgins and Anderson 1931; Michalopoulos and DeFrances 1997) or scarless wound healing in the oral mucosa (Sciubba et al. 1978; Szpaderska et al. 2003). In humans, there are very few examples of body parts comprised of multiple tissue types that are capable of innate regeneration; the digit tip proves to be a prime example of this phenomenon. Case reports reveal that human children can innately regenerate the digit tip, a complex tissue made up of bone, connective tissue, vasculature, nerves, epithelium, nail matrix, and nail plate (Fig. 1A; Douglas 1972; Illingworth 1974). Similarly, there is evidence that adults can regenerate the soft tissue and nail of the digit tip after amputation, but bone regeneration in adults has not been systematically assessed (Farell et al. 1977; Louis et al. 1980; Mennen and Wiese 1993). Other mammals, including mice, can also regenerate the digit tip (Borgens 1982; Singer et al. 1987); the mouse digit tip is anatomically analogous to the human digit tip and can regenerate at all ages (Fig. 1B; Douglas 1972; Borgens 1982; Neufeld and Zhao 1995). Both human and mouse digit tips are comprised of the terminal phalangeal bone, which is surrounded by connective tissue and a ventral fat pad, where sweat glands are located. The bone articulates with a more proximal phalangeal bone, forming a joint along with dorsal and ventral tendons. Blood vessels and nerves run along the lateral sides of the digit. Epithelium surrounds the digit, including the specialized nail epithelium, which is located dorsally and gives rise to the keratinized nail plate. The main anatomical differences between the human and mouse digit tip are digit bone shape, the presence of a sesamoid bone in the mouse digit, and the morphology of the nail plate (Fig. 1). The number of different tissues in the mouse digit tip, along with the suite of genetic and molecular tools available for mouse, makes it an ideal model to study composite tissue regeneration with the goal of translating findings to therapeutic interventions in humans.
Figure 1.
Anatomy of human and mouse digit tips. The human (A) and mouse (B) digit tips are morphologically similar, with the digit tip bone surrounded by blood vessels, nerves, and connective tissue and a ventral fat pad that houses sweat glands. The nail plate and nail epithelium are dorsal to the digit bone. Dorsal and ventral tendons attach to the digit bone. The dashed line in B indicates the amputation plane used in many digit tip regeneration studies.
Research into mouse digit tip regeneration has spanned decades and encompasses many different research modalities. In this review, we will discuss research into the origin, formation, and differentiation of the blastema, a structure that is essential to regeneration of many complex tissues and is still shrouded in mystery. We will also discuss the roles of distinct tissues in the mouse digit tip during regeneration including the bone, nail and nail epithelium, connective tissue, and nerves. An understanding of each tissue is important to grasp the holistic nature of complex tissue regeneration. Finally, regeneration of limbs and other appendages is often compared to the embryonic development of those structures, which can provide a framework to understanding differentiation of blastema cells and patterning of the regenerate. The fact that mouse digit tip regeneration occurs from fetal to adult stages enables a comparison of regeneration over the developmental time of a tissue. Collectively, we aim to provide a comprehensive summary of the current state of the field of mammalian digit tip regeneration and pose questions that could be addressed in future research.
THE MOUSE DIGIT TIP BLASTEMA
The term blastema is derived from the Greek word for offshoot or offspring. This naturally leads to the modern scientific definition of blastema: a mass of proliferating progenitor cells that is established in response to injury and eventually forms the regenerated tissue. However, a blastema forms in response to amputation in a wide range of regenerative model organisms, from planaria to mouse, thus mediating regeneration of tissues with different compositions and patterns; it remains to be determined how interrelated blastemas are between clades. Here, we will focus on what is known about the mouse digit tip blastema. Much of the recent research on the mouse digit tip blastema has focused on two intertwined questions: (1) origin: which cells give rise to the blastema, and (2) identity: which cells are present within the blastema.
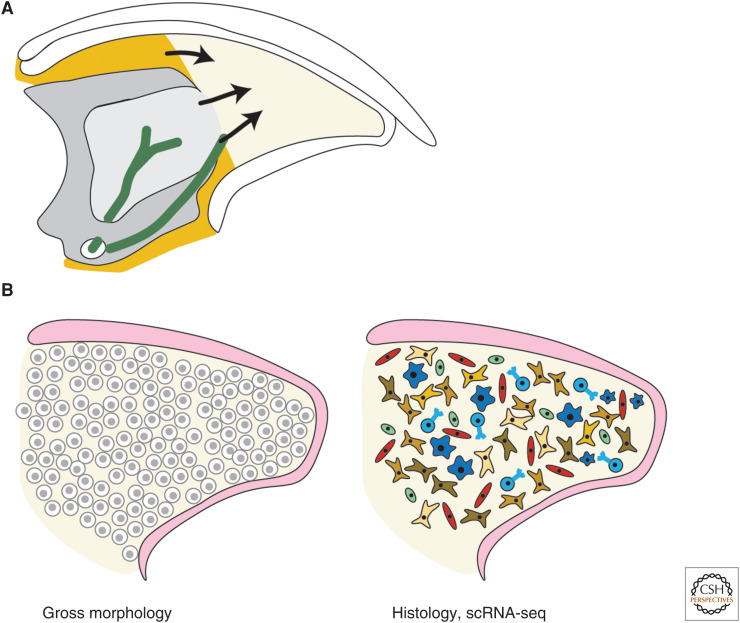
First, we will focus on the state of research into the origin of blastema cells. During mouse digit tip regeneration, the blastema forms shortly after the wound epithelium has closed over the amputation. This timing depends on the age and strain of the mouse, ranging from 7 days post- amputation (dpa) in the neonatal mouse (Han et al. 2008) to 10 dpa or later in the adult mouse (Fernando et al. 2011; Qu et al. 2020). Genetic lineage tracing approaches, where Cre recombinase is expressed under the control of a cell-type-specific promoter in combination with a Cre reporter allele to permanently mark cells and their progeny, have shown that there is likely not a multipotent progenitor cell that gives rise to all blastema cells (Lehoczky et al. 2011; Rinkevich et al. 2011); however, the exact origin of blastema cells remains unknown except in a few specific cases (Fig. 2A). For example, mesenchymal cells within peripheral nerves have been shown to contribute to the blastema, and Schwann cells within the blastema originate from de-differentiated peripheral nerve-associated Schwann cells (Johnston et al. 2016; Carr et al. 2019).
Figure 2.
Origin and identity of the blastema. (A) Schematic of tissues that give rise to blastema cells. Peripheral nerves (green) provide Schwann cells and mesenchymal cells. Pdgfra-expressing mesenchyme (orange) provides mesenchymal blastema cells. Bone marrow (light gray) and periosteum may also contribute cells to the blastema. (B) Evolution of the field's understanding of blastema identity over decades from a homogeneous, proliferative mass of cells defined based on gross section morphology (left), to a highly heterogeneous collection of cells through histology and single-cell RNA sequencing (scRNA-seq) analysis (right).
The origin of blastema cells is an inherently difficult problem to solve because blastema cells are defined by being present in the regenerative structure, but they must arise from digit stump tissue or the circulation. Parabiosis experiments, which permanently join the circulatory systems of two animals, have shown that circulating cells do not contribute to the blastema (Rinkevich et al. 2011), making digit stump tissue the most likely origin of blastema cells. One line of investigation has determined that the periosteum and bone marrow are the source of some blastema cells, based on histology that shows bone marrow and periosteal cells proliferating prior to blastema formation, and that removing the periosteum partially inhibits bone regeneration (Fig. 2A; Dawson et al. 2018). Future studies using genetic lineage tracing of periosteal and bone marrow populations could enrich our understanding of the dynamics and proportion of cells in the blastema derived from these tissues. A separate line of investigation addresses whether mesenchymal cells expressing Pdgfra contribute to the blastema. Genetic lineage tracing shows that Pdgfra-positive cells from the connective tissue and peripheral nerves do contribute to the blastema, and ablating Pdgfra-positive cells with a PdgfraCreERT;R26-LSL-DTA strategy impairs regeneration (Fig. 2A; Carr et al. 2019; Storer et al. 2020). Because Pdgfra is widely expressed in the connective tissue of the digit tip, these experiments do not define the origin of individual populations of fibroblasts. Determining the origin of blastema cells is an important question when considering how we could potentially induce complex tissue regeneration in humans. It is also becoming increasingly complicated as the heterogeneous nature of the blastema becomes clear, since there could be many different origins depending on the blastema cell type. DNA barcoding is conceptually similar to the above-mentioned genetic lineage tracing, but instead marks individual cells with permanent unique DNA sequence tags that persist in any daughter cells. Barcoding strategies can be used to reconstruct the lineage tree(s) of blastema cells and could be useful in defining the dynamics of blastema formation, for example, whether blastema cells arise from a few or many progenitors.
Several factors are known to play a role in the recruitment of blastema cells. Some cells in the blastema express CXCR4, a receptor for SDF-1 (Lee et al. 2013). SDF-1 is a chemokine that is expressed in the wound epithelium after amputation, implying that nascent blastema cells from throughout the digit dip may be attracted to the early wound epithelium through chemokine signaling (Lee et al. 2013). This is an attractive model that leverages the formation of a specialized wound epithelium after amputation. Experiments focused on inducing ectopic regeneration in digit regions that normally do not regenerate may also provide hints to the process of blastema formation. Bone morphogenetic protein (BMP) beads implanted into proximal amputation wounds can recruit cells that look histologically similar to a blastema and show some characteristics of the blastema, like proliferation and regeneration of bone (Yu et al. 2010). It would be interesting to characterize this ectopic tissue to determine how cellularly and molecularly related it is to the endogenous digit tip blastema.
Once the blastema is formed, it is accessible to techniques that allow definition of identity, or cell types, such as histology of cell markers. By gross morphology in tissue sections, the blastema appears as a homogeneous population of cells distal to the bone stump and enclosed by the wound epithelium (Fig. 2B) with visible blood vessels in later stages (Han et al. 2008; Fernando et al. 2011). Bone-associated markers such as Bmp4 and Runx2 are expressed in a subset of blastema cells (Han et al. 2008; Yu et al. 2010; Takeo et al. 2013). These genes make sense biologically since bone makes up most of the regenerated tissue in the mouse digit tip and BMPs and Runx2 are known to be involved in osteogenesis. As estimated from 5-ethynyl-2′-deoxyuridine (EdU) staining and single-cell sequencing experiments, ∼10% of all blastema cells are actively dividing (Johnston et al. 2016; Johnson et al. 2020; Storer et al. 2020). Genetic lineage tracing has shown that the cells of the mouse digit tip blastema are lineage restricted, at least to separate embryonic germ layer origin (Lehoczky et al. 2011; Rinkevich et al. 2011). For example, epithelial cells marked by K14 or K5 only contribute to regenerated epithelium (Lehoczky et al. 2011; Rinkevich et al. 2011). More recently, advances in defining the identity of blastema cells have been mediated by single-cell RNA sequencing (scRNA-seq), a technology that lends itself to cell-type identification. Our laboratory and others have used scRNA-seq to define the cell types present in the mouse digit tip blastema, including Schwann cells, immune cells such as macrophages, T cells, neutrophils, and monocytes, endothelial and vascular smooth muscle cells, and a heterogeneous population of fibroblasts (Fig. 2B, Table 1; Johnson et al. 2020; Storer et al. 2020). Some of these broad cell types were previously characterized in the blastema by histology with cell-type-specific markers, like Schwann cells and macrophages (Johnston et al. 2016; Simkin et al. 2017).
Table 1.
Reports defining specific cell types found in the blastema by cell-type-specific immunohistochemistry (IHC), RNA in situ hybridization (ISH), or single-cell transcriptomics (scRNA-seq)
| Cell type | References | Method |
|---|---|---|
| Fibroblast | Marrero et al. 2017 | IHC |
| Johnson et al. 2020 | scRNA-seq, FISH | |
| Storer et al. 2020 | scRNA-seq, FISH | |
| Osteoblast | Han et al. 2008 | RNA ISH |
| Yu et al. 2010 | RNA ISH | |
| Takeo et al. 2013 | IHC | |
| Johnson et al. 2020 | scRNA-seq | |
| Storer et al. 2020 | scRNA-seq | |
| Endothelial | Fernando et al. 2011 | IHC |
| Johnson et al. 2020 | scRNA-seq; | |
| Storer et al. 2020 | scRNA-seq | |
| Vascular smooth muscle | Johnson et al. 2020 | scRNA-seq |
| Storer et al. 2020 | scRNA-seq, IHC | |
| Schwann cell | Johnston et al. 2016 | IHC |
| Dolan et al. 2019 | IHC | |
| Johnson et al. 2020 | scRNA-seq | |
| Storer et al. 2020 | scRNA-seq, IHC | |
| Macrophage | Simkin et al. 2017 | IHC |
| Johnson et al. 2020 | scRNA-seq | |
| Storer et al. 2020 | scRNA-seq | |
| Monocyte | Johnson et al. 2020 | scRNA-seq |
| Neutrophil | Simkin et al. 2017 | IHC |
| Johnson et al. 2020 | scRNA-seq | |
| T-cell | Johnson et al. 2020 | scRNA-seq |
| Osteoclast | Johnson et al. 2020 | scRNA-seq |
| Storer et al. 2020 | scRNA-seq |
Many cell types like the ones mentioned above have well-defined markers, but the heterogeneity of some cell types, such as fibroblasts, has not always been appreciated. The connective tissue compartment of the digit tip is mainly made up of fibroblasts that were not well characterized until recently (Marrero et al. 2017; Johnson et al. 2020; Storer et al. 2020). Genetic lineage tracing using Msx1 or Prx1 Cre alleles showed that fibroblasts in the digit tip do not differentiate into other defined cell types like epithelium or endothelium (Lehoczky et al. 2011; Rinkevich et al. 2011). However, Msx1 and Prx1 are expressed widely in unamputated digit tip connective tissue and blastema cells, making it difficult to interrogate fibroblast subpopulations using existing genetic markers. Recent scRNA-seq studies have made headway in molecularly characterizing fibroblast heterogeneity in the blastema and unamputated digit tip (Johnson et al. 2020; Storer et al. 2020). These studies provide specific markers for several distinct fibroblast populations in the unamputated digit tip and the blastema, as well as some regeneration-specific fibroblast markers. The wealth of transcriptomic data now available at various regenerative time points can inform new genetic tools to trace populations more finely within the blastema, perhaps even allowing dissection of the origin and transdifferentiation of specific fibroblast populations. These defined blastema cell populations will also allow for examination of the spatial relationships of different cell types in the blastema. In future research, it will be important to frame the blastema as a heterogeneous structure with distinct cell populations that may contribute to regeneration in different ways.
DIGIT TIP BONE REGENERATION
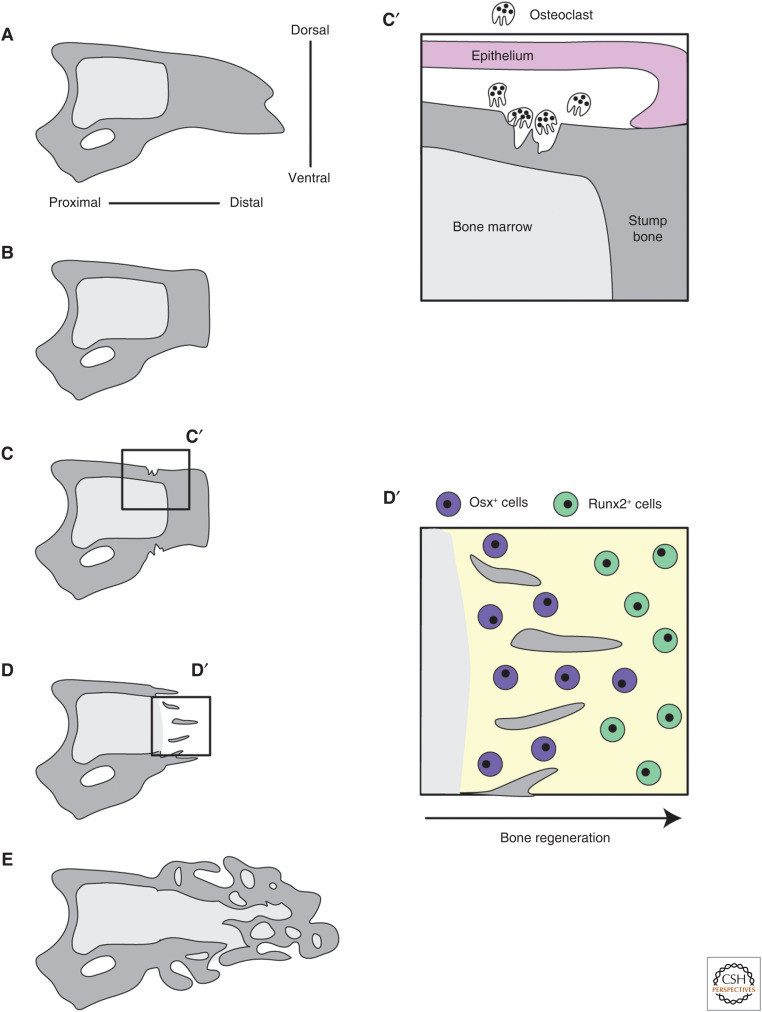
The terminal phalange bone (also referred to as the P3 bone) is a distinct anatomical feature of the mouse digit tip and has a characteristic triangular shape (Figs. 1B and 3A). The bone extends from the distal interphalangeal joint to near the distal end of the digit tip and is encased in connective tissue. Because the bone is the largest tissue in the digit tip and has long been used to quantify the amount of regeneration, the process of digit tip bone regeneration has been well studied and falls into distinct stages: amputation, bone histolysis, and bone differentiation (Fig. 3; Neufeld and Zhao 1995; Han et al. 2008; Fernando et al. 2011; Dawson et al. 2018).
Figure 3.
Digit tip bone regeneration. (A–E) A progression of digit tip bone regeneration following amputation. The intact bone (A) is amputated (B). Osteoclasts degrade the stump bone resulting in re-amputation of the bone that exposes the bone marrow cavity (C,C′). Bone begins to regenerate and proceeds from proximal to distal (D,D′). The regenerated bone is woven and does not perfectly regenerate the original bone shape (E). (C′) (Inset) Epithelial attachment and osteoclast activity. (D′) (Inset) The progression of bone regeneration from proximal to distal.
In digit amputations permissive for regeneration, up to 40% of the digit tip bone length is removed (Fig. 3A,B; Neufeld and Zhao 1995). Histology shows that following amputation, the wound epithelium adheres to the edges of the amputated bone instead of migrating or proliferating to close over it, leaving the stump bone exposed (Fernando et al. 2011; Simkin et al. 2017). This coincides with increased osteoclast activity near where the epithelium meets the stump bone (Fernando et al. 2011; Simkin et al. 2017). These osteoclasts degrade the stump bone and result in “re-amputation” of the bone whereby some of the remaining stump bone is degraded by histolysis and pushed out of the wound (Fig. 3C,C′). This phase of digit tip regeneration results in a closed wound and less than half of the terminal phalanx remaining, with the bone marrow cavity open to the wound environment. Interestingly, decoupling wound closure and histolysis by inducing wound closure over the stump bone shows that regeneration can occur in the absence of further bone degradation (Simkin et al. 2015).
Once the blastema has formed, osteoblast progenitors within the blastema begin to undergo osteoblastogenesis. Bone differentiation proceeds proximally to distally within the digit tip blastema. Osteoblasts near the stump express more mature osteoblasts markers (ex. Osx), and osteoblasts distal to the stump are more proliferative and express less mature osteoblast markers (ex. Runx2) (Fig. 3D,D′; Dawson et al. 2018). Digit bone regeneration occurs via intramembranous ossification and begins with woven bone islands near the stump, then proceeds distally until the entire digit tip bone is reconstituted (Han et al. 2008; Fernando et al. 2011). BMP signaling is necessary for bone regeneration, as Noggin application to a regeneration-permissive amputation can block the regeneration of new bone in fetal and neonatal mice (Han et al. 2003; Yu et al. 2010). Oxygen content of the tissue is also important for the process of digit tip bone regeneration. The early blastema is a hypoxic environment and increasing oxygen tension at this stage, prior to bone regeneration, causes a prolonged bone degradation period and increased variability in the timing of bone regrowth (Sammarco et al. 2014, 2015). Regeneration of the bone is imperfect: the volume of the regenerated bone eventually exceeds that of unamputated digit tip bones, and the new bone is woven instead of cortical (Fig. 3E; Han et al. 2008; Fernando et al. 2011). A recent quantitative analysis using microCT scans showed that the regenerated bone has a higher bone mineral density and a much larger bone marrow cavity than the unamputated bone (Hoffseth et al. 2021), although it is not known whether this affects the function of the regenerated digit tip. The regenerated bone also continues to be remodeled for some time after bone regeneration is considered complete, ∼4 weeks post-amputation (wpa) in adult mice.
Because bone regeneration has been well characterized, it is often used as the standard for completion of regeneration. This is useful because bone length and volume are easily quantifiable measures of digit tip regeneration. However, there are many tissues in the digit tip, such as nerves, blood vessels, and connective tissue. Moving forward, it would be informative to quantify the regeneration of these tissues as well in experiments assessing the completion or amount of regeneration following genetic perturbations or other manipulations, to ultimately understand the innate regeneration of composite tissue.
THE NAIL AND NERVES: SUPPORTERS OF REGENERATION
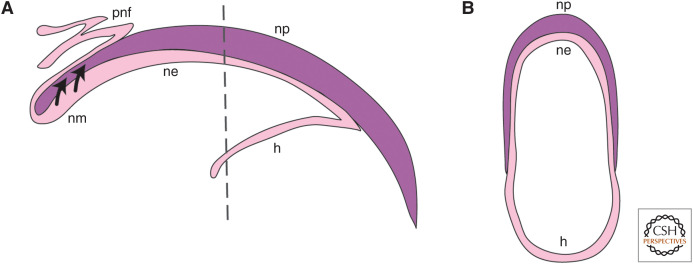
The nail is a structure that is integral to the digit tip. The nail itself is a plate comprised of hard keratin produced by the specialized nail matrix epithelial cells immediately below the proximal nail plate (Fig. 4; Nakamura and Ishikawa 2008; Takeo et al. 2013). Digit tip regeneration is known to be dependent on the amount of digit amputated, and successful regeneration only occurs for amputations transecting the nail; amputations proximal to the nail matrix do not regenerate (Fig. 1B; Neufeld and Zhao 1995). Furthermore, transplantation of the nail organ to a more proximal digital position can induce some bone regeneration following amputation (Zhao and Neufeld 1995). These early experiments suggested that the nail and nail matrix may play a role in creating a permissive environment for digit tip regeneration, and/or that nail stem cells contributed to the blastema. K14 and K5 genetic lineage tracing experiments conclude that it is unlikely that nail stem cells are contributing to the cellular composition of the blastema (Lehoczky et al. 2011; Rinkevich et al. 2011), although they could give rise to the cells of the wound epithelium. This supports the hypothesis that the nail matrix supplies pro-regenerative signals after amputation.
Figure 4.
Anatomy of the nail organ. (A) A transverse section through the digit tip showing the nail plate (np), nail matrix (nm), nail epithelium (ne), proximal nail fold (pnf), and hyponychium (h). Nail stem cells contribute to the np (arrows). Proximal is to the left and distal to the right. (B) A dorsal–ventral cross section through the digit at the plane of the dashed line in A showing the np, ne, and h, curving around the digit. Dorsal is to the top and ventral to the bottom in A and B.
How might the nail matrix contribute to a regenerative environment in the digit tip? In both adult and neonatal mice, the nail matrix is a Wnt signaling center (Takeo et al. 2013; Lehoczky and Tabin 2015). Lgr6, a Wnt signaling agonist, is expressed in the proximal nail matrix and marks nail stem cells (Lehoczky and Tabin 2015). The digits of Lgr6 knockout mice develop normally, but display a regeneration-specific phenotype of non-regenerative nails at a reduced penetrance, and significantly smaller regenerated bones as compared to wild-type controls (Lehoczky and Tabin 2015). There is also evidence that Wnt signaling in the nail matrix may define the regenerative boundary (Takeo et al. 2013). It has been demonstrated that Wnt signaling is active in the distal nail matrix but not the proximal nail matrix where nail stem cells are located. Conditional knockout of β-catenin in the epithelium results in loss of nail growth as well as deficient nail and bone regeneration after amputation (Takeo et al. 2013). Interestingly, activating Wnt signaling by conditionally overexpressing β-catenin in the epithelium of a proximal non-regenerative digit amputation leads to some nerve, bone, and nail regrowth, as well as cellular proliferation. This only occurs if the amputation is distal enough to leave some nail matrix intact, which implies that Wnt signaling specifically from nail matrix cells is indeed necessary for the regenerative response (Takeo et al. 2013).
These two studies together point to Wnt signaling in the nail matrix as a necessary part of digit tip regeneration, but the exact mechanism remains unknown. For example, how does the nail epithelium signal to underlying tissue? There is evidence that nail and bone homeostasis are disrupted by abrogation of Wnt signaling in the nail matrix, which leads to changes in Wnt signaling in osteoblasts and osteoclasts associated with the underlying digit bone, but the mechanism remains to be determined (Takeo et al. 2016). Furthermore, early experiments with nail ablation and transplantation did not differentiate between the nail matrix and the nail plate (Fig. 4; Neufeld and Zhao 1995; Zhao and Neufeld 1995). These studies show that it is likely that the nail matrix molecularly supports regeneration, but there is no direct evidence that excludes a role for the nail plate itself. The nail plate is a hard structure that surrounds most of the dorsal and lateral digit tip, raising the possibility that the nail plate itself could provide mechanical feedback that affects regeneration, including the resulting bone shape, although this remains to be formally explored.
Another tissue that is essential in supporting digit regeneration is the peripheral nerve. Peripheral nerves provide support for blastema-mediated limb regeneration in salamanders in the form of mitogens (Singer and Craven 1948; Egar 1988; Kumar et al. 2007) and have also been a focus of research in digit tip regeneration. The digit tip is innervated by the digital nerve, which branches in two at the proximal end of the digit tip bone. One branch enters the bone marrow of the P3 bone and is comprised of sympathetic axons, while the other branch surrounds the P3 bone with both sensory and sympathetic axons (Fig. 1B; Dolan et al. 2019). After amputation, Schwann cells are present in the blastema and axons are found in the area around the blastema (Johnston et al. 2016; Dolan et al. 2019). Interestingly, at 4 wpa, the nerve structure found in the unamputated digit tip has not completely regenerated. There are fewer axons in the regenerated digit tip as well as fewer myelinating Schwann cells associated with axons as measured by immunohistochemistry for axon (β3T) and Schwann cell (GFAP) markers (Dolan et al. 2019). It has been noted that some regenerated digits do not regenerate sensory axons at all; even at 120 dpa, ∼17 wpa experiments reveal that the level of β3T expression is not returned to pre-amputation levels. This implies that the axons are not simply slower to regenerate than other digit tip structures—but that they may never regenerate completely (Dolan et al. 2019). One interesting follow-up question is whether the reduction in axons and Schwann cells affects the overall functionality of the digit tip nerves, leading to decreased function in the digit.
Reports on the effects of denervation on regeneration are varied (Table 2). Denervation of the sciatic nerve in rats was first shown to not have a significant effect on bone regeneration as measured by length at 4 wpa, although wound healing and nail regrowth were delayed (Mohammad and Neufeld 2000). However, separate sciatic nerve denervation experiments in mice do report a decrease in bone and nail regeneration after denervation at 4 wpa (Johnston et al. 2016) or 5 wpa (Takeo et al. 2013). In mice where the sciatic and femoral nerves were resected, bone and nail at 3 months post-amputation regenerate but show aberrant morphology (Rinkevich et al. 2014). In another variation on denervation, Dolan et al. amputate digits once and then re-amputate at 4 wpa, when nerves are not completely regenerated. They find no defect in bone regeneration after this re-amputation, even though the nerves have not regenerated to pre-amputation levels (Dolan et al. 2019). This is distinct from complete denervation of the sciatic nerve and raises questions about denervation of a very specific anatomical area versus general denervation. This range of outcomes likely reflects differences in the age of the mouse and/or the timing between denervation and amputation (Table 2), and because bone and nail morphology are assessed as regeneration endpoints, it is unclear how denervation effects regeneration of other tissues. Bone and nail are not the only players in regeneration, and it would be informative to characterize the response of additional tissues as well to broaden our understanding of which tissues peripheral nerves influence directly or indirectly.
Table 2.
Summary of experiments testing the necessity of nerves for digit tip regeneration
| References | Nerve | Age at denervation | Timing of amputation | Regeneration defect |
|---|---|---|---|---|
| Mohammad and Neufeld 2000 | Sciatic | 17 d | Concurrent with denervation | None at 4 wpa |
| Takeo et al. 2013 | Sciatic | 2 wk | 7 d after denervation | Bone and nail length reduced at 5 wpa |
| Rinkevich et al. 2014 | Sciatic and femoral | Likely ≥3 wk | 14 d after denervation | Regenerated bone disorganized at 3 mpa |
| Johnston et al. 2016 | Sciatic | 10–12 wk | 10 d after denervation | Bone area and nail length reduced at 4 wpa |
| Dolan et al. 2019 | N/A | N/A | 4 wk after first amputation | None at 4 wpa (second amputation) |
Despite the varied results from denervation, the data reveal that nerves provide support to digit tip regeneration in the form of mitogens and cells. Schwann cells are present in the blastema but not associated with nerves (Johnston et al. 2016). When Schwann cells are genetically ablated, the regenerated bone and nail show defects, recapitulating the regeneration phenotypes resulting from denervation (Johnston et al. 2016). Certain factors, including PDGFAA and OSM, are expressed by blastemal Schwann cells and can rescue denervation induced regeneration defects in the bone and nail. This is compelling evidence that nerve-associated cells can affect the blastema environment to promote regeneration. Furthermore, mesenchymal cells from the nerve itself can enter the blastema and contribute to regeneration of the bone (Carr et al. 2019). These results show that the nerve plays a role in supporting digit tip regeneration and opens the door to further investigation into how Schwann cells and neural crest–derived mesenchymal cells are activated and recruited by injury to contribute to regeneration.
DIGIT TIP REGENERATION IN THE CONTEXT OF LIMB DEVELOPMENT
In species capable of complete limb regeneration, like salamanders, regeneration appears morphologically similar to embryonic limb development (Goss 1969). The cellular processes are also comparable at a gross level: cells proliferate to form the blastema, which will differentiate to form the regenerate similar to the formation and differentiation of the embryonic limb bud mesenchyme. The wound epithelium is necessary for blastema formation in mouse digit tip regeneration (Simkin et al. 2017), just as the apical ectodermal ridge is for limb development (Saunders 1948; Summerbell 1974). There are two questions related to development that can be asked in the context of digit tip regeneration: how does development or maturation of the tissue affect the process of regeneration, and does regeneration molecularly recapitulate embryonic development of the limb or digit tip?
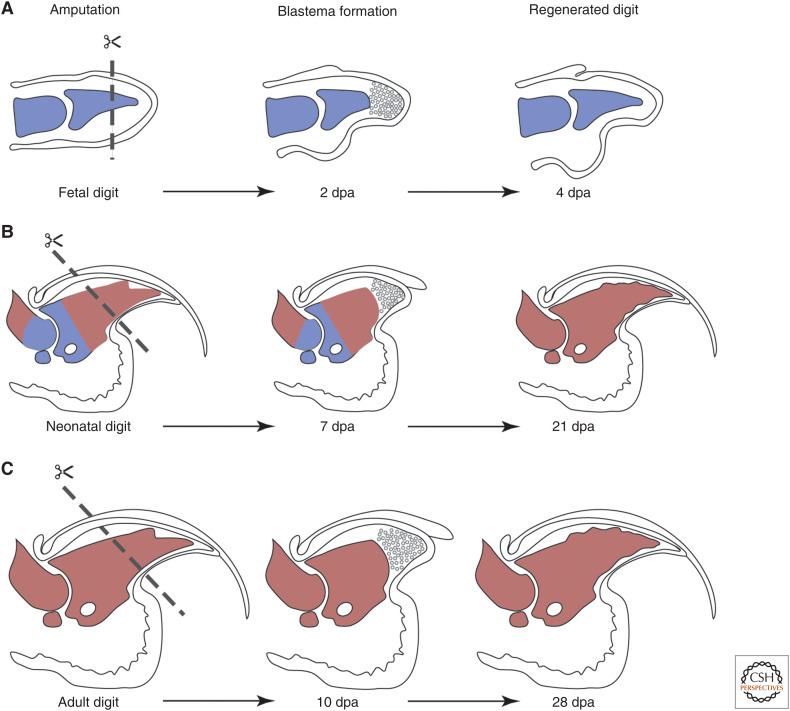
The question of how age affects the process of digit tip regeneration is interesting because its impact on efficacy might vary among mammalian species. Mice of all ages can regenerate digit tips. In humans, children can regenerate soft tissue and bone (Douglas 1972; Illingworth 1974), while in adults, digit bone regeneration has not been comprehensively evaluated compared to pediatric patients (McKim 1932; Farell et al. 1977; Louis et al. 1980; Singer et al. 1987). Digit tip regeneration in fetal or neonatal mice is faster than regeneration in adult mice (Han et al. 2003, 2008; Fernando et al. 2011); whether this reflects differences in the rate of proliferation and differentiation, differences in the regenerating tissue volumes, or both, remains to be explored. Whereas fetal mice can regenerate amputated digit tips in 3–4 days, neonatal mice generally take 21 days and adult mice 28 days, using the bone as a reference (Fig. 5; Han et al. 2003, 2008; Fernando et al. 2011). In fetal mice, the boundary of Msx1 and Msx2 expression correlates with the amputation plane that will result in regeneration, and this appears to continue throughout neonatal and adult stages (Reginelli et al. 1995; Han et al. 2003). The tissues involved are also different between fetal and neonatal or adult mice. For example, the embryonic skeleton is still chondrogenic whereas the neonatal bones are ossifying, and the adult digit tip bone is completely mineralized (Fig. 5). Broadly speaking, neonatal and adult digit tips are morphologically very similar and contain the same types of tissues, although adult digit tips are made up of terminally differentiated cells while neonatal digit tips are still rapidly growing. Fetal digit tips are rapidly growing and composed of undifferentiated mesenchyme. Whether maturation of the digit tissues affects the process of regeneration is an intriguing question, as it could provide hints to why more mature tissues regenerate more slowly or not at all.
Figure 5.
Development and digit tip regeneration. (A) Digit tip amputation at the fetal stage (left) transects the cartilaginous digit, undifferentiated mesenchyme, and epithelium. The blastema has formed at 2 d post-amputation (dpa) (center) and regeneration is complete at 4 dpa (right). (B) In neonates, amputation transects ossifying bone that is still growing, the nail and epithelium, and connective tissue (left). The blastema forms by 7 dpa (center) and the digit bone is regenerated by 21 dpa (right). (C) In adults, amputation transects fully calcified bone, the nail and epithelium, and connective tissue (left). The blastema forms by 10 dpa (center) and the digit bone is regenerated by 28 dpa (right). Red denotes bone and blue denotes cartilage. Digits are not drawn to scale.
The second question pertaining to embryonic development and digit tip regeneration involves determining whether developmental patterning genes and pathways are reused for tissue patterning during regeneration. For example, in non-mammalian regenerative models such as frogs and salamanders, key developmental patterning genes such as Shh, Fgfs, and some members of the Hox gene family are reexpressed during limb or tail regeneration in patterns similar to limb or tail development (Torok et al. 1999; Endo et al. 2000; Carlson et al. 2001; Roensch et al. 2013). In mouse digit tip regeneration however, there are limited studies addressing re-deployment of embryonic limb development pathways. Recent scRNA-seq of the digit tip blastema and the embryonic mouse limb bud showed that limb bud and blastema mesenchymal cells are not transcriptionally equivalent, but they do partially overlap in gene expression space (Storer et al. 2020), indicating that some limb development genes are important to blastema cell identity. Moreover, RNA FISH reveals that the distal limb patterning gene Hoxa13 is expressed in the blastema (Qu et al. 2020), which also supports the idea that developing limb and blastema cells share some important regulators.
One clear example of digit tip regeneration differing from its initial development occurs in bone. During embryonic development, the digit tip bone is formed by endochondral ossification, whereby first a cartilage template of the bone is formed, and then hypertrophic chondrocytes are replaced by osteogenic cells that differentiate and mineralize. Unlike other long bones, the digit tip bone only has one growth plate on the proximal end. The bone is formed by endochondral ossification but grows after birth by appositional ossification (Casanova and Sanz-Ezquerro 2007). In contrast, the digit tip bone regenerates through intramembranous or direct ossification, which does not involve chondrocytes (Han et al. 2008). Curiously though, bone regeneration induced by a BMP7 bead in a proximal amputation was reported to occur via endochondral ossification, implying that both processes are possible in the regenerating digit tip (Yu et al. 2010). Endochondral and intramembranous ossification are well-characterized processes that facilitate robust comparison of digit tip bone development and regeneration. However, there are other tissues within the digit tip, including nerves, blood vessels, and fibroblasts that go through their own developmental processes and stages. Investigation into whether these tissues follow embryonic molecular pathways during regeneration would add to overall understanding of digit tip regeneration and whether it recapitulates aspects of embryonic limb development.
There are many open questions in this area that remain to be investigated. Transcriptional comparisons of fetal, neonatal, and adult blastemas, at the single-cell level or with bulk RNA-seq, could provide insight into how regeneration proceeds at different stages of maturation. Candidate gene approaches investigating genes that are well characterized in development in the context of digit tip regeneration can show whether these genes are necessary during regeneration, and in what process (for example, in patterning of the regenerate, or cell proliferation, etc.). Another direction would be comparing gene regulation between limb or digit development and regeneration, which could provide an evolutionary dimension to the concept of developmental genes being redeployed during regeneration. The same genes could be expressed but from a different regulatory context. Overall, studying the potential connection of embryonic limb development to digit tip regeneration will provide important context and insight into the process that can ultimately inform translational medicine efforts.
CONCLUDING REMARKS
In this review, we present a comprehensive overview of the field of digit tip regeneration by discussing the blastema and the roles of various tissues in formation and differentiation of this regeneration-specific structure. There are several exciting new avenues that promise to offer more insight into the process of mammalian digit tip regeneration. For example, the presence of immune cell types such as macrophages and neutrophils have been characterized with histology (Simkin et al. 2017), but we lack an understanding of how these cell types interact with the digit tip tissues, including the blastema, before or during regeneration. In particular, how the wound healing and inflammation phase of digit tip regeneration is resolved and transitioned to the regenerative phase is an important area of future study.
Another tissue that is important for regeneration in other models is the wound epithelium. In salamander, the wound epithelium is a specialized structure that is necessary for regeneration. The wound epithelium signals to the underlying blastema (Boilly and Albert 1990; Christensen et al. 2002), and removing it inhibits regeneration (Thornton 1957; Mescher 1976). To date, there has been limited investigation directly into the mouse digit tip wound epithelium; histology has been used to determine the timing of the wound epithelium closure, and forcing early wound closure shows that the wound epithelium interacts with the bone histolysis phase of regeneration (Simkin et al. 2015), but little molecular characterization has been reported. Perhaps most important is to determine whether there is a specialized wound epithelium, or whether the nail epithelium takes over this role in the digit tip. This would minimally require molecular and transcriptomic characterization and comparison of the regenerative wound epithelium and the homeostatic nail epithelium.
Finally, the blastema is arguably the most important structure in digit tip regeneration, but also the most mysterious. This makes it difficult to determine whether models of induced proximal regeneration (such as proximal nail grafting or BMP7 bead implantation [Mohammad et al. 1999; Yu et al. 2010]) are mediated by formation of a blastema that is the same as is formed during innate digit tip regeneration or not. Classically, proliferation and gross morphology of the tissue have been used to determine whether a structure is a blastema, but growing evidence for the heterogeneity of the mammalian blastema argues that these features are not enough to define a blastema (Fig. 2B). A new generation of single-cell genomic tools provides an exciting opportunity for further epigenetic, transcriptomic, and lineage characterization of the blastema, which combined with characterization of induced blastema structures, will clarify our understanding of the identity of the blastema. The ultimate goal of inducing blastema-mediated regeneration in humans relies on translation of the principles of regeneration found in animal models, and the mouse digit tip will continue to provide valuable insight into complex tissue regeneration.
ACKNOWLEDGMENTS
Supported by funding from the National Institutes of Health (NIH) R03HD093922, R21HD097405, and funds from Brigham and Women's Hospital (BWH) Department of Orthopedic Surgery (to J.A.L).
Footnotes
Editors: Kenneth D. Poss and Donald T. Fox
Additional Perspectives on Regeneration available at www.cshperspectives.org
REFERENCES
- Boilly B, Albert P. 1990. In vitro control of blastema cell proliferation by extracts from epidermal cap and mesenchyme of regenerating limbs of axolotls. Rouxs Arch Dev Biol 198: 443–447. 10.1007/BF00399054 [DOI] [PubMed] [Google Scholar]
- Borgens R. 1982. Mice regrow the tips of their foretoes. Science 217: 747–750. 10.1126/science.7100922 [DOI] [PubMed] [Google Scholar]
- Carlson MRJ, Komine Y, Bryant SV, Gardiner DM. 2001. Expression of Hoxb13 and Hoxc10 in developing and regenerating axolotl limbs and tails. Dev Biol 229: 396–406. 10.1006/dbio.2000.0104 [DOI] [PubMed] [Google Scholar]
- Carr MJ, Toma JS, Johnston APW, Steadman PE, Yuzwa SA, Mahmud N, Frankland PW, Kaplan DR, Miller FD. 2019. Mesenchymal precursor cells in adult nerves contribute to mammalian tissue repair and regeneration. Cell Stem Cell 24: 240–256.e9. 10.1016/j.stem.2018.10.024 [DOI] [PubMed] [Google Scholar]
- Casanova JC, Sanz-Ezquerro JJ. 2007. Digit morphogenesis: is the tip different? Dev Growth Differ 49: 479–491. 10.1111/j.1440-169X.2007.00951.x [DOI] [PubMed] [Google Scholar]
- Christensen RN, Weinstein M, Tassava RA. 2002. Expression of fibroblast growth factors 4, 8, and 10 in limbs, flanks, and blastemas of Ambystoma. Dev Dyn 223: 193–203. 10.1002/dvdy.10049 [DOI] [PubMed] [Google Scholar]
- Dawson LA, Schanes PP, Kim P, Imholt FM, Qureshi O, Dolan CP, Yu L, Yan M, Zimmel KN, Falck AR, et al. 2018. Blastema formation and periosteal ossification in the regenerating adult mouse digit. Wound Repair Regen 26: 263–273. 10.1111/wrr.12666 [DOI] [PubMed] [Google Scholar]
- Dolan CP, Yan M, Zimmel K, Yang TJ, Leininger E, Dawson LA, Muneoka K. 2019. Axonal regrowth is impaired during digit tip regeneration in mice. Dev Biol 445: 237–244. 10.1016/j.ydbio.2018.11.010 [DOI] [PubMed] [Google Scholar]
- Douglas BS. 1972. Conservative management of guillotine amputation of the finger in children. J Paediatr Child Health 8: 86–89. 10.1111/j.1440-1754.1972.tb01793.x [DOI] [PubMed] [Google Scholar]
- Egar MW. 1988. Accessory limb production by nerve-induced cell proliferation. Anat Rec 221: 550–564. 10.1002/ar.1092210111 [DOI] [PubMed] [Google Scholar]
- Endo T, Tamura K, Ide H. 2000. Analysis of gene expressions during Xenopus forelimb regeneration. Dev Biol 220: 296–306. 10.1006/dbio.2000.9641 [DOI] [PubMed] [Google Scholar]
- Farell R, Disher W, Nesland R, Palmatier T, Truhler T. 1977. Conservative management of fingertip amputations. J Am Coll Emerg Physicians 6: 243–246. 10.1016/S0361-1124(77)80461-9 [DOI] [PubMed] [Google Scholar]
- Fernando WA, Leininger E, Simkin J, Li N, Malcom CA, Sathyamoorthi S, Han M, Muneoka K. 2011. Wound healing and blastema formation in regenerating digit tips of adult mice. Dev Biol 350: 301–310. 10.1016/j.ydbio.2010.11.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goss RJ. 1969. Principles of regeneration. Elsevier, Amsterdam. [Google Scholar]
- Han M, Yang X, Farrington JE, Muneoka K. 2003. Digit regeneration is regulated by Msx1 and BMP4 in fetal mice. Development 130: 5123–5132. 10.1242/dev.00710 [DOI] [PubMed] [Google Scholar]
- Han M, Yang X, Lee J, Allan CH, Muneoka K. 2008. Development and regeneration of the neonatal digit tip in mice. Dev Biol 315: 125–135. 10.1016/j.ydbio.2007.12.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins G, Anderson R. 1931. Experimental pathology of the liver: restoration of the liver of the white rat following partial surgical removal. Arch Pathol 12: 186–202. [Google Scholar]
- Hoffseth KF, Simkin J, Busse E, Stewart K, Watt J, Chapple A, Hargrove A, Sammarco MC. 2021. A new approach to analyzing regenerated bone quality in the mouse digit amputation model using semi-automatic processing of microCT data. Bone 144: 115776. 10.1016/j.bone.2020.115776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Illingworth CM. 1974. Trapped fingers and amputated finger tips in children. J Pediatr Surg 9: 853–858. 10.1016/S0022-3468(74)80220-4 [DOI] [PubMed] [Google Scholar]
- Johnson SL, Weston JA. 1995. Temperature-sensitive mutations that cause stage-specific defects in zebrafish fin regeneration. Genetics 141: 1583–1595. 10.1093/genetics/141.4.1583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson GL, Masias EJ, Lehoczky JA. 2020. Cellular heterogeneity and lineage restriction during mouse digit tip regeneration at single-cell resolution. Dev Cell 52: 525–540.e5. 10.1016/j.devcel.2020.01.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston APW, Yuzwa SA, Carr MJ, Mahmud N, Storer MA, Krause MP, Jones K, Paul S, Kaplan DR, Miller FD. 2016. Dedifferentiated Schwann cell precursors secreting paracrine factors are required for regeneration of the mammalian digit tip. Cell Stem Cell 19: 433–448. 10.1016/j.stem.2016.06.002 [DOI] [PubMed] [Google Scholar]
- Kumar A, Godwin JW, Gates PB, Garza-Garcia AA, Brockes JP. 2007. Molecular basis for the nerve dependence of limb regeneration in an adult vertebrate. Science 318: 772–777. 10.1126/science.1147710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Marrero L, Yu L, Dawson LA, Muneoka K, Han M. 2013. SDF-1α/CXCR4 signaling mediates digit tip regeneration promoted by BMP-2. Dev Biol 382: 98–109. 10.1016/j.ydbio.2013.07.020 [DOI] [PubMed] [Google Scholar]
- Lehoczky JA, Tabin CJ. 2015. Lgr6 marks nail stem cells and is required for digit tip regeneration. Proc Natl Acad Sci 112: 13249–13254. 10.1073/pnas.1518874112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehoczky JA, Robert B, Tabin CJ. 2011. Mouse digit tip regeneration is mediated by fate-restricted progenitor cells. Proc Natl Acad Sci 108: 20609–20614. 10.1073/pnas.1118017108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis DS, Palmer AK, Burney RE. 1980. Open treatment of digital tip injuries. J Am Med Assoc 244: 697–698. 10.1001/jama.1980.03310070047031 [DOI] [PubMed] [Google Scholar]
- Marrero L, Simkin J, Sammarco M, Muneoka K. 2017. Fibroblast reticular cells engineer a blastema extracellular network during digit tip regeneration in mice. Regeneration 4: 69–84. 10.1002/reg2.75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKim LH. 1932. Regeneration of the distal phalanx. Can Med Assoc J 46: 549–550. [PMC free article] [PubMed] [Google Scholar]
- Mennen U, Wiese A. 1993. Fingertip injuries management with semi-occlusive dressing. J Hand Surg Br 18: 416–422. 10.1016/0266-7681(93)90139-7 [DOI] [PubMed] [Google Scholar]
- Mescher AL. 1976. Effects on adult newt limb regeneration of partial and complete skin flaps over the amputation surface. J Exp Zool 195: 117–127. 10.1002/jez.1401950111 [DOI] [PubMed] [Google Scholar]
- Michalopoulos GK, DeFrances MC. 1997. Liver regeneration. Science 276: 60–66. 10.1126/science.276.5309.60 [DOI] [PubMed] [Google Scholar]
- Mohammad KS, Neufeld DA. 2000. Denervation retards but does not prevent toetip regeneration. Wound Repair Regen 8: 277–281. 10.1046/j.1524-475x.2000.00277.x [DOI] [PubMed] [Google Scholar]
- Mohammad KS, Day FA, Neufeld DA. 1999. Bone growth is induced by nail transplantation in amputated proximal phalanges. Calcif Tissue Int 65: 408–410. 10.1007/s002239900722 [DOI] [PubMed] [Google Scholar]
- Nakamura M, Ishikawa O. 2008. The localization of label-retaining cells in mouse nails. J Invest Dermatol 128: 728–730. 10.1038/sj.jid.5701062 [DOI] [PubMed] [Google Scholar]
- Neufeld DA, Zhao W. 1995. Bone regrowth after digit tip amputation in mice is equivalent in adults and neonates. Wound Repair Regen 3: 461–466. 10.1046/j.1524-475X.1995.30410.x [DOI] [PubMed] [Google Scholar]
- Poss KD, Wilson LG, Keating MT. 2002. Heart regeneration in zebrafish. Science 298: 2188–2190. 10.1126/science.1077857 [DOI] [PubMed] [Google Scholar]
- Qu F, Palte IC, Gontarz PM, Zhang B, Guilak F. 2020. Transcriptomic analysis of bone and fibrous tissue morphogenesis during digit tip regeneration in the adult mouse. FASEB J 34: 9740–9754. 10.1096/fj.202000330R [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reginelli AD, Wang YQ, Sassoon D, Muneoka K. 1995. Digit tip regeneration correlates with regions of Msx1 (Hox 7) expression in fetal and newborn mice. Development 121: 1065–1076. 10.1242/dev.121.4.1065 [DOI] [PubMed] [Google Scholar]
- Rinkevich Y, Lindau P, Ueno H, Longaker MT, Weissman IL. 2011. Germ-layer and lineage-restricted stem/progenitors regenerate the mouse digit tip. Nature 476: 409–413. 10.1038/nature10346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinkevich Y, Montoro DT, Muhonen E, Walmsley GG, Lo D, Hasegawa M, Januszyk M, Connolly AJ, Weissman IL, Longaker MT. 2014. Clonal analysis reveals nerve-dependent and independent roles on mammalian hind limb tissue maintenance and regeneration. Proc Natl Acad Sci 111: 9846–9851. 10.1073/pnas.1410097111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roensch K, Tazaki A, Chara O, Tanaka EM. 2013. Progressive specification rather than intercalation of segments during limb regeneration. Science 342: 1375–1379. 10.1126/science.1241796 [DOI] [PubMed] [Google Scholar]
- Sammarco MC, Simkin J, Fassler D, Cammack AJ, Wilson A, Van Meter K, Muneoka K. 2014. Endogenous bone regeneration is dependent upon a dynamic oxygen event. J Bone Miner Res 29: 2336–2345. 10.1002/jbmr.2261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sammarco MC, Simkin J, Cammack AJ, Fassler D, Gossmann A, Marrero L, Lacey M, Van Meter K, Muneoka K. 2015. Hyperbaric oxygen promotes proximal bone regeneration and organized collagen composition during digit regeneration. PLoS ONE 10: 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders JW. 1948. The proximo-distal sequence of origin of the parts of the chick wing and the role of the ectoderm. J Exp Zool 108: 363–403. 10.1002/jez.1401080304 [DOI] [PubMed] [Google Scholar]
- Sciubba JJ, Waterhouse JP, Meyer J. 1978. A fine structural comparison of the healing of incisional wounds of mucosa and skin. J Oral Pathol Med 7: 214–227. 10.1111/j.1600-0714.1978.tb01596.x [DOI] [PubMed] [Google Scholar]
- Simkin J, Sammarco MC, Dawson LA, Tucker C, Taylor LJ, Van Meter K, Muneoka K. 2015. Epidermal closure regulates histolysis during mammalian (Mus) digit regeneration. Regeneration 2: 106–119. 10.1002/reg2.34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simkin J, Sammarco MC, Marrero L, Dawson LA, Yan M, Tucker C, Cammack A, Muneoka K. 2017. Macrophages are required to coordinate mouse digit tip regeneration. Development 144: 3907–3916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer M, Craven L. 1948. The growth and morphogenesis of the regenerating forelimb of adult Triturus following denervation at various stages of development. J Exp Zool 108: 279–308. 10.1002/jez.1401080207 [DOI] [PubMed] [Google Scholar]
- Singer M, Weckesser EC, Géraudie J, Maier CE, Singer J. 1987. Open finger tip healing and replacement after distal amputation in Rhesus monkey with comparison to limb regeneration in lower vertebrates. Anat Embryol (Berl) 177: 29–36. 10.1007/BF00325287 [DOI] [PubMed] [Google Scholar]
- Storer MA, Mahmud N, Karamboulas K, Borrett MJ, Yuzwa SA, Gont A, Androschuk A, Sefton MV, Kaplan DR, Miller FD. 2020. Acquisition of a unique mesenchymal precursor-like blastema state underlies successful adult mammalian digit tip regeneration. Dev Cell 52: 509–524.e9. 10.1016/j.devcel.2019.12.004 [DOI] [PubMed] [Google Scholar]
- Summerbell D. 1974. A quantitative analysis of the effect of excision of the AER from the chick limb-bud. J Embryol Exp Morphol 32: 651–660. [PubMed] [Google Scholar]
- Szpaderska AM, Zuckerman JD, DiPietro LA. 2003. Differential injury responses in oral mucosal and cutaneous wounds. J Dent Res 82: 621–626. 10.1177/154405910308200810 [DOI] [PubMed] [Google Scholar]
- Takeo M, Chou WC, Sun Q, Lee W, Rabbani P, Loomis C, Taketo MM, Ito M. 2013. Wnt activation in nail epithelium couples nail growth to digit regeneration. Nature 499: 228–232. 10.1038/nature12214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeo M, Hale CS, Ito M. 2016. Epithelium-derived Wnt ligands are essential for maintenance of underlying digit bone. J Invest Dermatol 136: 1355–1363. 10.1016/j.jid.2016.03.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton CS. 1957. The effect of apical cap removal on limb regeneration in Amblystoma larvae. J Exp Zool 134: 357–381. 10.1002/jez.1401340209 [DOI] [PubMed] [Google Scholar]
- Torok MA, Gardiner DM, Izpisúa-Belmonte JC, Bryant SV. 1999. Sonic Hedgehog (SHH) expression in developing and regenerating axolotl limbs. J Exp Zool 284: 197–206. [PubMed] [Google Scholar]
- Yu L, Han M, Yan M, Lee EC, Lee J, Muneoka K. 2010. BMP signaling induces digit regeneration in neonatal mice. Development 137: 551–559. 10.1242/dev.042424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao W, Neufeld DA. 1995. Bone regrowth in young mice stimulated by nail organ. J Exp Zool 271: 155–159. 10.1002/jez.1402710212 [DOI] [PubMed] [Google Scholar]