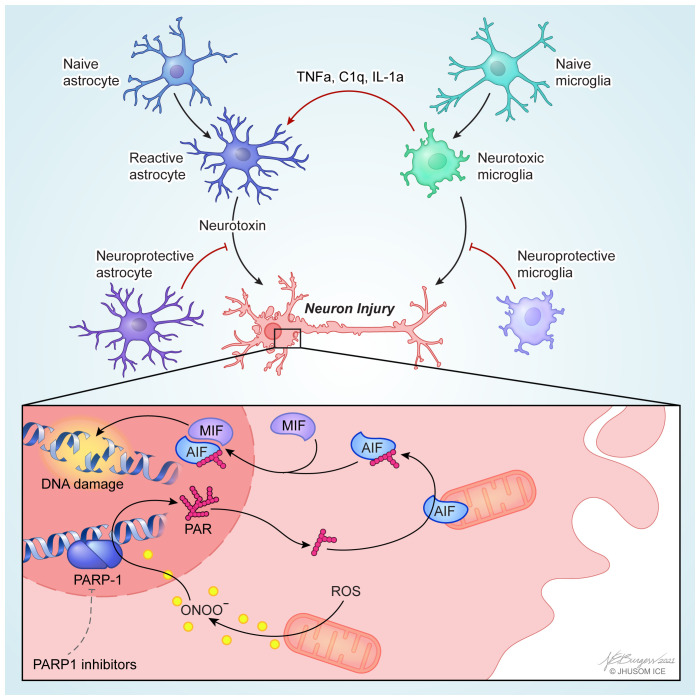
Figure 1. Cell-autonomous and non-cell-autonomous neurodegeneration.
PARP1-dependent cell-autonomous mechanisms of neurodegeneration (bottom). Neuron injury stressors such as an oxidative stress or aggregated proteins activate nitric oxide synthase that produces nitric oxide and then peroxynitrite (ONOO−), resulting in overactivation of PARP1. Accumulated poly (ADP-ribose) (PAR) polymers synthesized by overactivated PARP1 translocate from the nucleus to the cytoplasm and mitochondria, where it binds to and induces mitochondrial release of apoptosis-inducing factor (AIF). AIF-bound macrophage migration-inducing factor (MIF) nuclease translocates into the nucleus, where MIF cleaves genomic DNA into large-scale fragments, causing cell death. Inhibition of PARP1 can protect neurons in a variety of neurodegenerative diseases (see ‘Prevention of cell-autonomous neurodegeneration’ section). Non-cell-autonomous mechanisms of neurodegeneration mediated by microglia or astrocytes (top). Induction of disease-associated microglia or homeostatic microglia and subsequent prevention of neurotoxic microglia could be promising neuroprotection strategies in neurodegenerative diseases. Alternatively, activated microglia induces the formation of neurotoxic reactive astrocytes by secreting interleukin 1α (IL-1α), tumor necrosis factor α (TNF-α), and C1q. Reactive astrocyte-targeted neuroprotection could be achieved by microglial inhibition of formation of neurotoxic reactive astrocytes and induction of neuroprotective astrocytes. PARP, poly (ADP-ribose) polymerase; ROS, reactive oxygen species.